Abstract
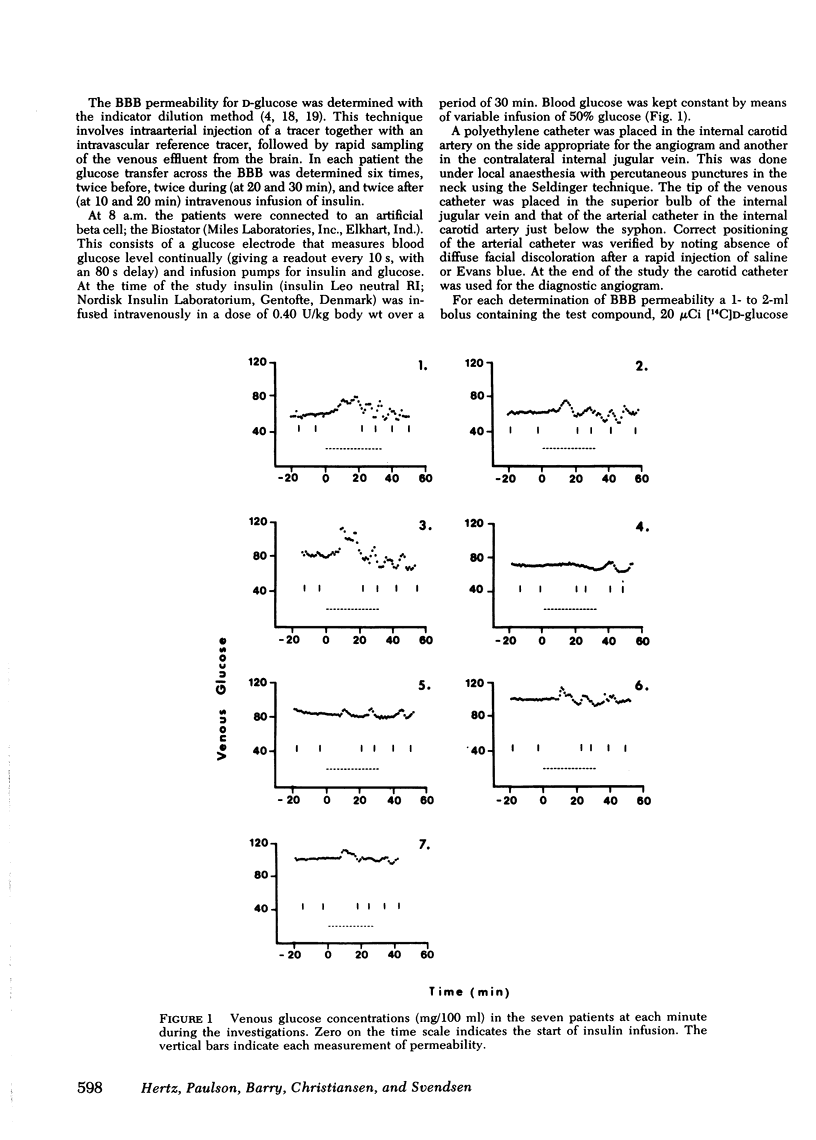
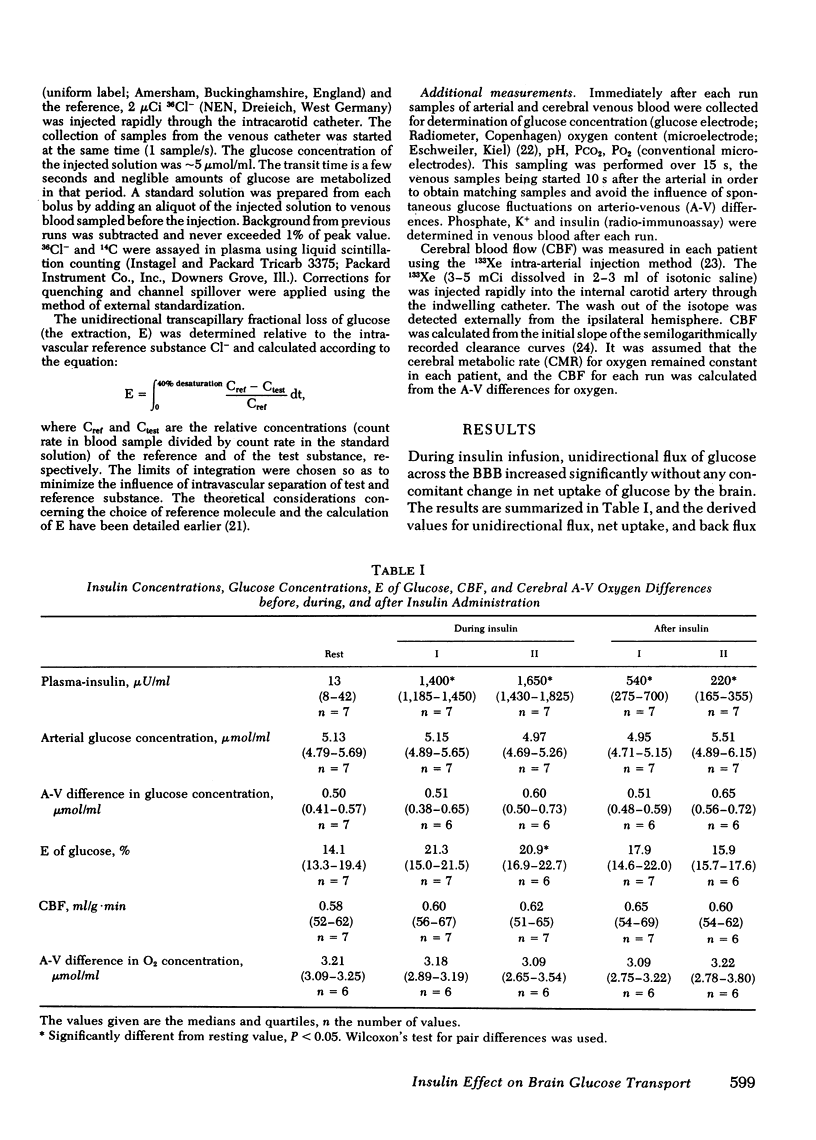
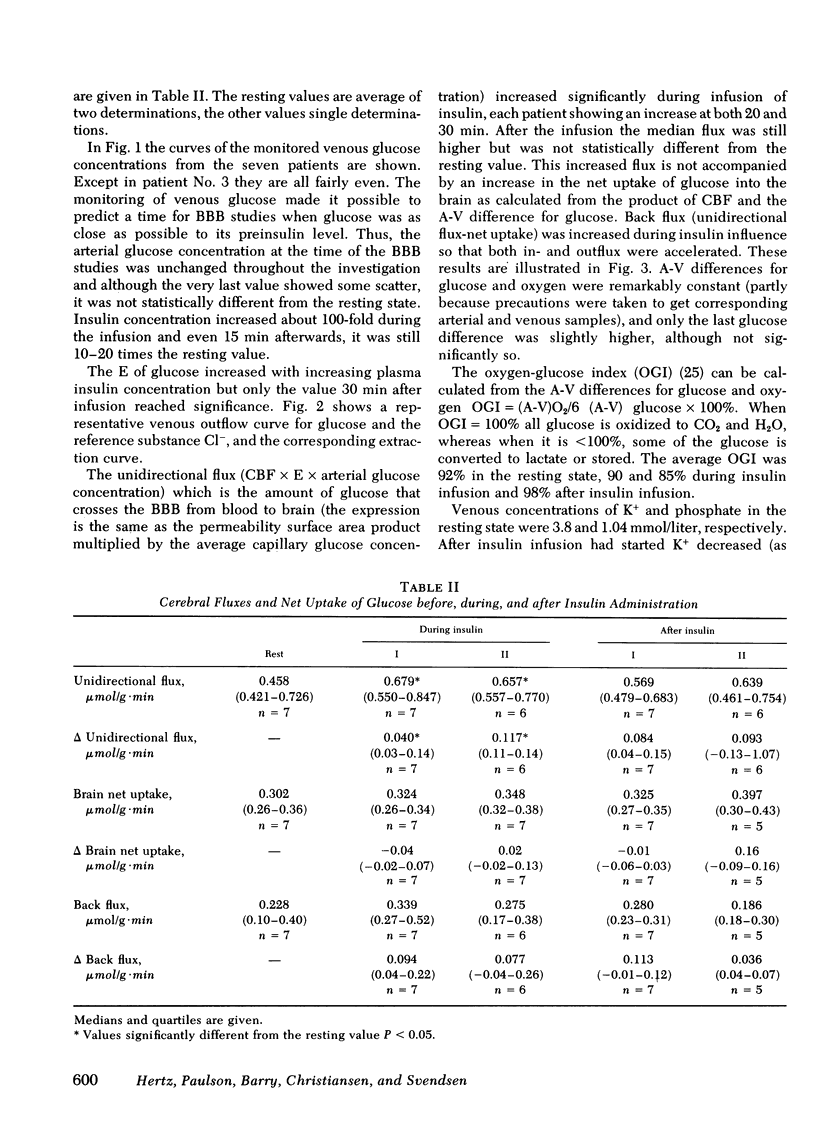
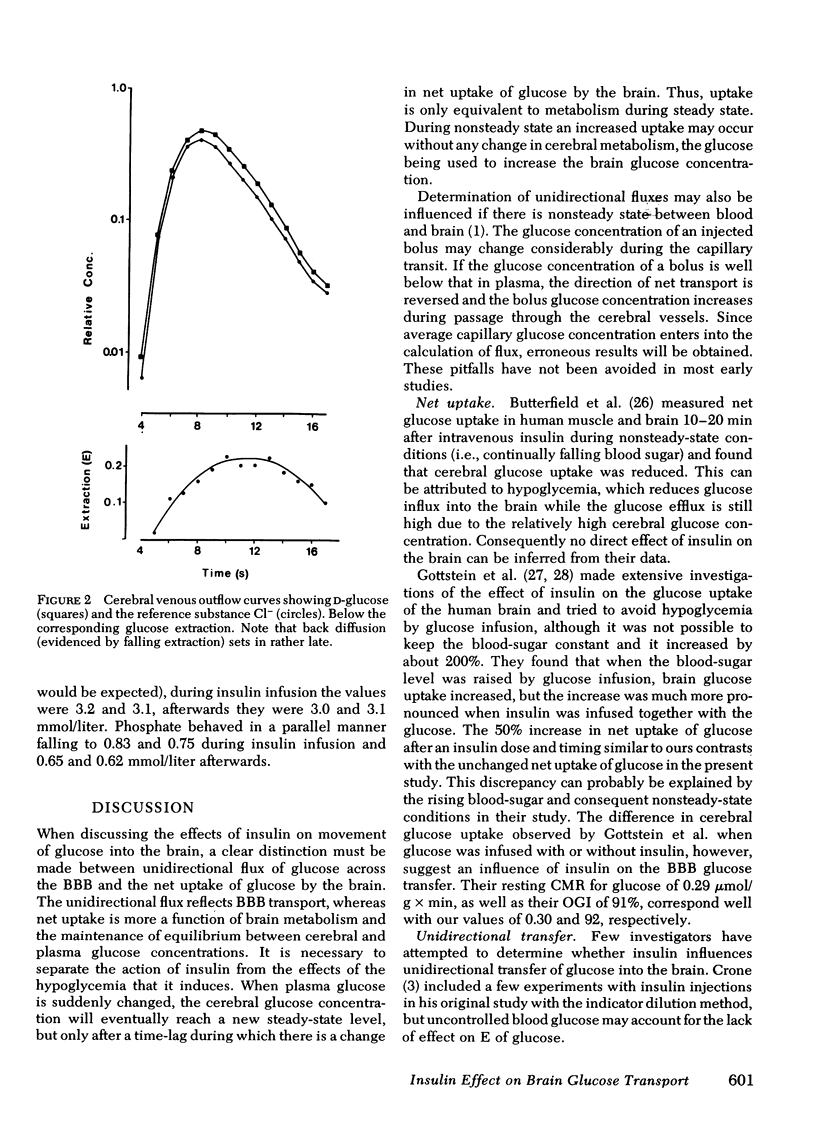
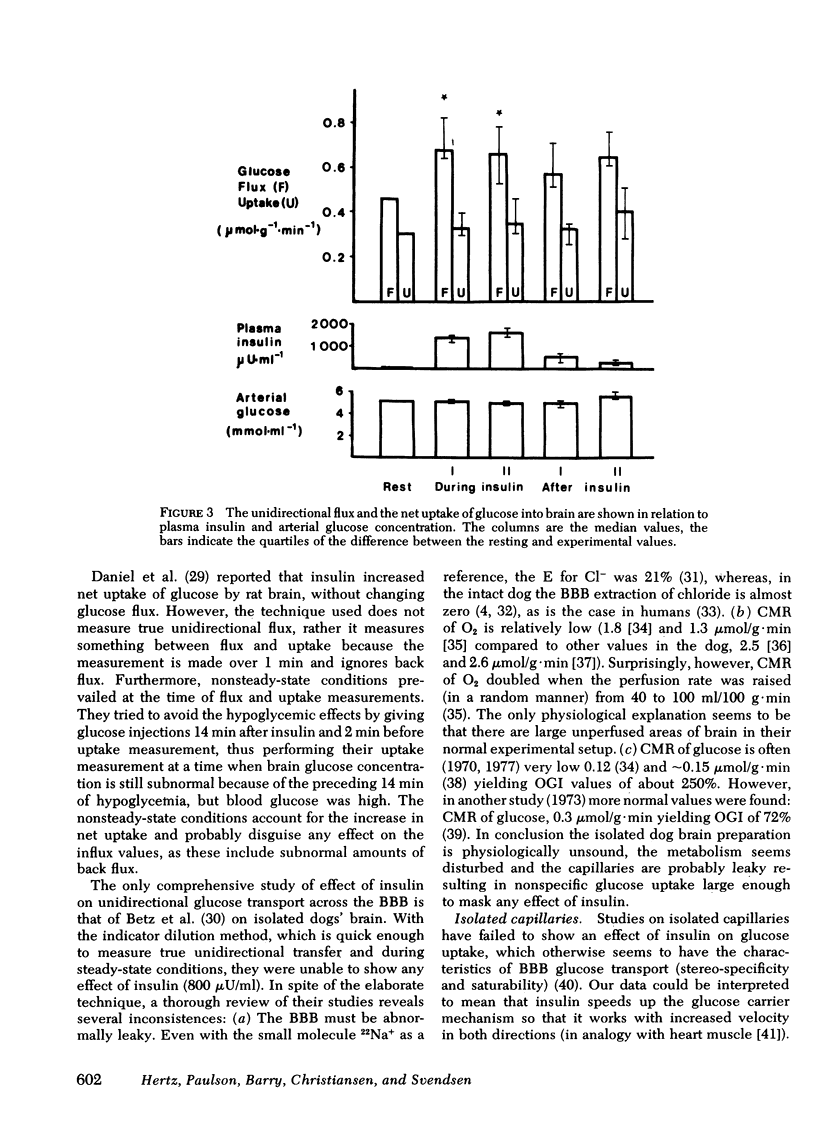
The influence of insulin on unidirectional flux of glucose across the blood-brain barrier and on net uptake of glucose by the brain was investigated in seven fasting patients. The unidirectional extraction, E, of [14C]D-glucose was determined using 36Cl- as an intravascular reference, by the indicator dilution method. 0.4 U insulin/kg body wt was infused intravenously over 30 min while blood glucose was maintained constant by glucose infusion. Six determinations were made in each patient, two before, two during insulin infusion, and two after. In connection with each blood-brain barrier study, arterial and cerebral venous samples were taken for measurement of glucose, oxygen, insulin, K+, and phosphate. Cerebral blood flow (CBF) was measured in each patient. The main finding was an increased extraction of glucose from 14 to 21% and a highly significant increase in unidirectional flux (CBF X unidirectional extraction X arterial glucose concentration) from 0.46 to 0.66 mumol/g X min during insulin infusion (plasma insulin approximately 1,500 microU/ml). The net brain uptake of glucose (CBF X arterio-venous difference for glucose) as unaltered during the investigation period of 45 min, which is too short a time for insulin to penetrate the barrier. It follows that the backflux of glucose from the brain was increased during insulin application. The effect of insulin might be a speeding up of the glucose carrier in analogy to heart muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Rahman A., Agardh C. D., Siesjø B. K. Local cerebral blood flow in the rat during severe hypoglycemia, and in the recovery period following glucose injection. Acta Physiol Scand. 1980 Jul;109(3):307–314. doi: 10.1111/j.1748-1716.1980.tb06601.x. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S., Daniel P. M., Love E. R., Pratt O. E. The transport of glucose into the brain of the rat in vivo. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):71–82. doi: 10.1098/rspb.1973.0005. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Csejtey J., Goldstein G. W. Hexose transport and phosphorylation by capillaries isolated from rat brain. Am J Physiol. 1979 Jan;236(1):C96–102. doi: 10.1152/ajpcell.1979.236.1.C96. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Yudilevich D. L., Drewes L. R. Kinetics of unidirectional glucose transport into the isolated dog brain. Am J Physiol. 1973 Sep;225(3):586–592. doi: 10.1152/ajplegacy.1973.225.3.586. [DOI] [PubMed] [Google Scholar]
- Blennow G., Folbergrová J., Nilsson B., Siesjö B. K. Effects of bicuculline-induced seizures on cerebral metabolism and circulation of rats rendered hypoglycemic by starvation. Ann Neurol. 1979 Feb;5(2):139–151. doi: 10.1002/ana.410050207. [DOI] [PubMed] [Google Scholar]
- Bolwig T. G., Hertz M. M., Paulson O. B., Spotoft H., Rafaelsen O. J. The permeability of the blood-brain barrier during electrically induced seizures in man. Eur J Clin Invest. 1977 Apr;7(2):87–93. doi: 10.1111/j.1365-2362.1977.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Borgström L., Hägerdal M., Lewis L., Pontén U. Polarographic determination of total oxygen content in small blood samples. Scand J Clin Lab Invest. 1974 Dec;34(4):375–380. doi: 10.3109/00365517409049894. [DOI] [PubMed] [Google Scholar]
- Butterfield W. J., Abrams M. E., Sells R. A., Sterky G., Whichelow M. J. Insulin sensitivity of the human brain. Lancet. 1966 Mar 12;1(7437):557–560. doi: 10.1016/s0140-6736(66)90757-4. [DOI] [PubMed] [Google Scholar]
- COHEN P. J., WOLLMAN H., ALEXANDER S. C., CHASE P. E., BEHAR M. G. CEREBRAL CARBOHYDRATE METABOLISM IN MAN DURING HALOTHANE ANESTHESIA: EFFECTS OF PACO2 ON SOME ASPECTS OF CARBOHYDRATE UTILIZATION. Anesthesiology. 1964 Mar-Apr;25:185–191. doi: 10.1097/00000542-196403000-00013. [DOI] [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Conover C., Regen D. M., Whitfield C. F., Morgan H. E. Effect of insulin on kinetics of sugar transport in heart muscle. Am J Physiol. 1978 Jan;234(1):E70–E78. doi: 10.1152/ajpendo.1978.234.1.E70. [DOI] [PubMed] [Google Scholar]
- Clemens A. H., Chang P. H., Myers R. W. The development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS). Horm Metab Res. 1977;Suppl 7:23–33. [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Thompson A. M. Comparative studies of capillary permeability in brain and muscle. Acta Physiol Scand. 1973 Feb;87(2):252–260. doi: 10.1111/j.1748-1716.1973.tb05388.x. [DOI] [PubMed] [Google Scholar]
- Cutler R. W., Sipe J. C. Mediated transport of glucose between blood and brain in the cat. Am J Physiol. 1971 May;220(5):1182–1186. doi: 10.1152/ajplegacy.1971.220.5.1182. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Pratt O. E. Insulin and the way the brain handles glucose. J Neurochem. 1975 Oct;25(4):471–476. doi: 10.1111/j.1471-4159.1975.tb04352.x. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Pratt O. E. The influence of insulin upon the metabolism of glucose by the brain. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):85–104. doi: 10.1098/rspb.1977.0031. [DOI] [PubMed] [Google Scholar]
- Drewes L. R., Gilboe D. D. Glycolysis and the permeation of glucose and lactate in the isolated, perfused dog brain during anoxia and postanoxic recovery. J Biol Chem. 1973 Apr 10;248(7):2489–2496. [PubMed] [Google Scholar]
- Drewes L. R., Gilboe D. D. Nutrient transport systems in dog brain. Fed Proc. 1977 Feb;36(2):166–170. [PubMed] [Google Scholar]
- Drewes L. R., Horton R. W., Betz A. L., Gilboe D. D. Cytochalasin B inhibition of brain glucose transport and the influence of blood components on inhibitor concentration. Biochim Biophys Acta. 1977 Dec 15;471(3):477–486. doi: 10.1016/0005-2736(77)90051-7. [DOI] [PubMed] [Google Scholar]
- Gilboe D. D., Andrews R. L., Dardenne G. Factors affecting glucose uptake by the isolated dog brain. Am J Physiol. 1970 Sep;219(3):767–773. doi: 10.1152/ajplegacy.1970.219.3.767. [DOI] [PubMed] [Google Scholar]
- Gilboe D. D., Betz A. L. Oxygen uptake in the isolated canine brain. Am J Physiol. 1973 Mar;224(3):588–595. doi: 10.1152/ajplegacy.1973.224.3.588. [DOI] [PubMed] [Google Scholar]
- Gottstein U., Held K., Sebening H., Walpurger G. Der Glucoseverbrauch des menschlichen Gehirns unter dem Einfluss intravenöser Infusionen von Glucose, Glucagon und Glucose-Insulin. Klin Wochenschr. 1965 Sep 15;43(18):965–975. doi: 10.1007/BF01747857. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Schmechel D., Roth J., Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz M. M., Paulson O. B. Heterogeneity of cerebral capillary flow in man and its consequences for estimation of blood-brain barrier permeability. J Clin Invest. 1980 May;65(5):1145–1151. doi: 10.1172/JCI109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedt-Rasmussen K., Sveinsdottir E., Lassen N. A. Regional cerebral blood flow in man determined by intra-arterial injection of radioactive inert gas. Circ Res. 1966 Mar;18(3):237–247. doi: 10.1161/01.res.18.3.237. [DOI] [PubMed] [Google Scholar]
- Lassen N. A., Trap-Jensen J., Alexander S. C., Olesen J., Paulson O. B. Blood-brain barrier studies in man using the double-indicator method. Am J Physiol. 1971 Jun;220(6):1627–1633. doi: 10.1152/ajplegacy.1971.220.6.1627. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967 Sep 23;215(5108):1375–1376. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- Mellerup E. T., Rafaelsen O. J. Brain glycogen after intracisternal insulin injection. J Neurochem. 1969 May;16(5):777–781. doi: 10.1111/j.1471-4159.1969.tb06456.x. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Nelson S. R., Schulz D. W., Passonneau J. V., Lowry O. H. Control of glycogen levels in brain. J Neurochem. 1968 Nov;15(11):1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M. Ability of circulating insulin to chronically regulate the cellular glucose transport system. Metabolism. 1978 Dec;27(12 Suppl 2):1917–1929. doi: 10.1016/s0026-0495(78)80009-2. [DOI] [PubMed] [Google Scholar]
- Olesen J., Paulson O. B., Lassen N. A. Regional cerebral blood flow in man determined by the initial slope of the clearance of intra-arterially injected 133Xe. Stroke. 1971 Nov-Dec;2(6):519–540. doi: 10.1161/01.str.2.6.519. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Setchell B. P. Cerebral glucose transport and oxygen consumption in sheep and rabbits. J Physiol. 1973 Sep;233(3):529–551. doi: 10.1113/jphysiol.1973.sp010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFAELSEN O. J. Action of insulin on glucose uptake of rat brain slices and isolated rat cerebellum. J Neurochem. 1961 Apr;7:45–51. doi: 10.1111/j.1471-4159.1961.tb13496.x. [DOI] [PubMed] [Google Scholar]
- Theye R. A., Michenfelder J. D. The effect of nitrous oxide on canine cerebral metabolism. Anesthesiology. 1968 Nov-Dec;29(6):1119–1124. doi: 10.1097/00000542-196811000-00007. [DOI] [PubMed] [Google Scholar]
- Vinten J., Gliemann J., Osterlind K. Exchange of 3-O-methylglucose in isolated fat cells. Concentration dependence and effect of insulin. J Biol Chem. 1976 Feb 10;251(3):794–800. [PubMed] [Google Scholar]
- Woods S. C., Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol. 1977 Oct;233(4):E331–E334. doi: 10.1152/ajpendo.1977.233.4.E331. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., De Rose N. Blood-brain transfer of glucose and other molecules measured by rapid indicator dilution. Am J Physiol. 1971 Mar;220(3):841–846. doi: 10.1152/ajplegacy.1971.220.3.841. [DOI] [PubMed] [Google Scholar]
- van Houten M., Posner B. I. Insulin binds to brain blood vessels in vivo. Nature. 1979 Dec 6;282(5739):623–625. doi: 10.1038/282623a0. [DOI] [PubMed] [Google Scholar]


