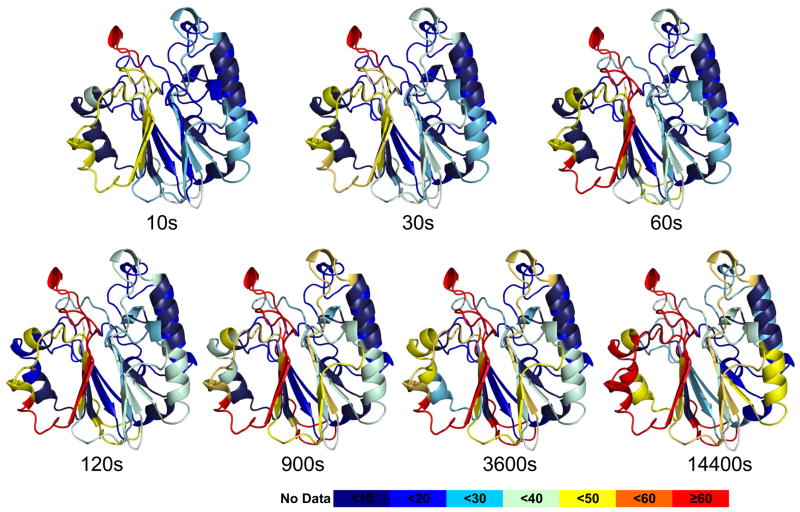
Figure 1.
HDX exchange data mapped on the structure of APE1 is consistent with a fully-folded protein. A. The percentage of deuterium uptake for peptides derived from APE1 at different time points are color coded on a ribbon rendering of the crystal structure of human APE1 (PDB:1BIX). The color code explained at the bottom indicates the deuterium uptake level with red indicative of regions that exchange very rapidly and dark blue regions that exchange very slowly. For the most rapidly exchanging peptides, 266–271 and 267– 273, greater than 70% of the backbone amide hydrogens exchanged with deuterium within 10 s. This region was also identified as interacting with E3330.