Figure 4. The role of residues contributing to substrate-binding cavities.
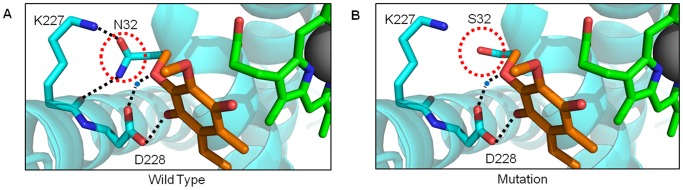
MT-CYB has several deep pockets for binding, redox interaction and modification of substrates. (A) The Qi site is formed by the contribution of multiple helical regions (blue) that fold around heme b H (green) while maintaining contact with the solvent. The wild-type residue N32 (PDB 1NTZ) is shown making hydrogen bonds (dotted lines) with the natural substrate ubiquinone (orange), within the same pocket. (B) Mutation to S32 results in the loss of key hydrogen bonds previously identified to be crucial in the redox mechanism.
