Abstract
Background
Postoperative pain after TKA is a major concern to patients. The best technique to control pain is still controversial. Intrathecal morphine or periarticular multimodal drug injection are both commonly used and both appear to provide better pain control than placebo, but it is unclear whether one or the other provides better pain control.
Questions/purposes
We asked whether intrathecal morphine or periarticular multimodal drug injection provides better pain control with fewer adverse events.
Methods
In a prospective, double-blind, randomized controlled trial we randomized 57 patients with osteoarthritic knees who underwent TKAs into two groups. Group M (n = 28) received 0.2 mg intrathecal morphine while Group I (n = 29) received periarticular multimodal drug injection. Postoperative pain was managed with patient-controlled analgesia using ketorolac. The outcomes were pain levels, the amount of analgesic drug used, and drug-related side effects. Patients and evaluators were blinded. All patients were followed up to 3 months.
Results
We found no difference in postoperative pain level, analgesia drug consumption, blood loss in drain, and knee function. More patients in Group M required antiemetic (19 [69%] versus 10 [34%]) and antipruritic drugs (10 [36%] versus three [10%]) than patients in Group I.
Conclusions
The two techniques provide no different pain control capacity. The periarticular multimodal drug injection was associated with lower rates of vomiting and pruritus.
Level of Evidence
Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA has been recognized as a successful procedure for treatment of osteoarthritic knees. Effective pain control improved postoperative rehabilitation and knee flexion have been reported [21]. Multiple analgesic techniques have been used for patients undergoing TKA such as intrathecal morphine [6, 12], epidural block [16], femoral nerve block [19, 24], intraarticular drug infusion [11], and periarticular multimodal drug injection (anesthetic cocktail) [3, 25].
Overusing narcotic drugs for postoperative pain management is associated with side effects including nausea, vomiting, pruritus, urinary retention, respiratory depression, and cognitive change [15]. Intrathecal morphine reportedly controls pain in patients undergoing TKA [6, 12], although there are concerns of morphine-related side effects [12]. Periarticular multimodal drug injection is being used more frequently and several studies [3, 18, 20, 25] showed its ability to provide postoperative pain control and reduced narcotic-related side effects by minimizing narcotic consumption. Both techniques control pain better than a placebo [3, 6, 25]. However, it is unclear whether one technique provides better pain control than the other.
We therefore asked whether intrathecal morphine or periarticular multimodal drug injection provides better pain control with fewer adverse events.
Patients and Methods
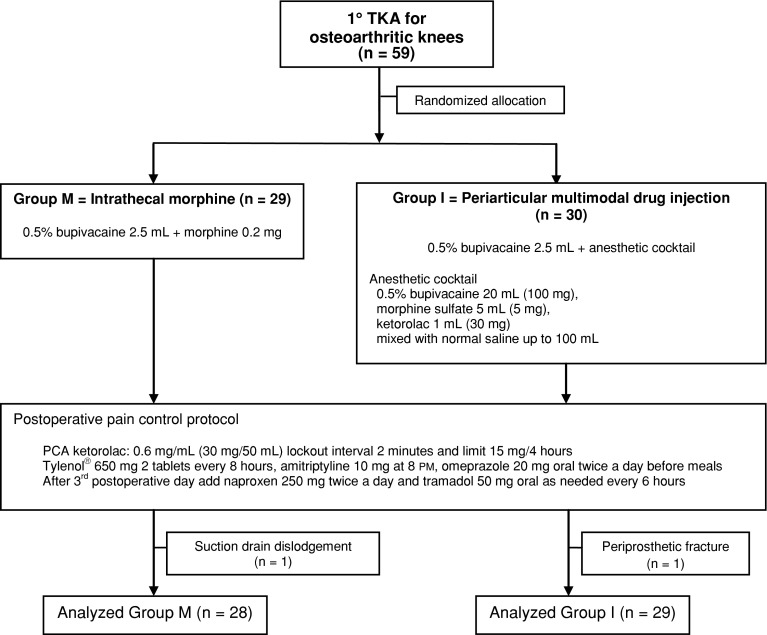
We prospectively evaluated 81 patients to enroll in a double-blind, randomized controlled trial of patients undergoing primary TKA from July 2010 to May 2011. The inclusion criteria were a diagnosis of primary osteoarthritis of the knee, younger than 80 years, good mentality, and patients agreed to participate. We excluded 22 patients for one of the following reasons: allergy to any drugs used in this study, severe liver or renal insufficiency, history of stroke or coronary heart disease, and cognitive impairment. These exclusions left 59 patients who underwent 59 TKAs. Two knees were excluded from analysis because one had drain dislodgement before 48 hours and the other had a periprosthetic fracture (Lewis and Rorabeck Type 2) from an accident 6 weeks after surgery. The patient’s fracture was fixed with a distal femoral locking plate. Consequently, 57 knees in 57 patients (46 females, 11 males) were included for analysis.
Sample size analysis was calculated. Fifty-four knees were required to detect a difference in VAS pain score greater than 20 points, with a SD of approximately 26 points in each group [7]. A two-sided hypothesis test at an alpha level of 0.05 and power of 80% was used. Ten percent dropout was added to confirm sufficient power to detect the difference calculated. Thus, 59 patients (59 knees) were recruited into the study.
Computerized block randomization was done by an independent research assistant (TY) who otherwise was not engaged in the study. The anesthesiologist assistant nurse who opened sealed opaque envelopes containing the randomization result allocated patients into each group. There were 28 patients in Group M and 29 patients in Group I (Fig. 1). We observed no difference for demographic data and preoperative clinical conditions (Table 1).
Fig. 1.
The flow chart shows the protocol of this study. PCA = patient-controlled analgesia.
Table 1.
Demographic data and preoperative clinical conditions*
| Data | Group M (n = 28) | Group I (n = 29) | p value |
|---|---|---|---|
| Demographic data | |||
| Gender (female/male) | 20/8 | 26/3 | – |
| Age (years) | 69 (8) | 70 (7) | 0.44 |
| Height (cm) | 159 (8) | 155 (7) | 0.11 |
| Weight (kg) | 69 (14) | 69 (13) | 0.95 |
| BMI (kg/m2) | 27 (5) | 29 (6) | 0.37 |
| Preoperative clinical conditions | |||
| Motion arc | |||
| Flexion (degrees) | 127 (13) | 121 (16) | 0.11 |
| Number of flexion contractures | 8 | 7 | – |
| Flexion contracture (degrees) | 9 (9) | 6 (2) | 0.4 |
| Number of recurvatum | 2 | 3 | – |
| Recurvatum (degrees) | 8 (4) | 8 (3) | 0.79 |
| Deformity | |||
| Number of varus | 26 | 27 | – |
| Varus (degrees-MA) | 11 (4) | 12 (4) | 0.51 |
| Number of valgus | 2 | 2 | – |
| Valgus (degrees-AA) | 13 (1) | 13 (6) | 1 |
| Modified WOMAC™ score | 50 (13) | 43 (15) | 0.08 |
* Data presented as means with SD in parentheses, except for gender, number of flexion contractures or recurvatum and number of varus or valgus deformities, which are presented as numbers; Group M = intrathecal morphine; Group I = periarticular multimodal drug injection; MA = mechanical axis; AA = anatomic axis.
All patients had the same preoperative multimodal pain control protocol consisting of 0.5 mg lorazepam (Utopian, Samutprakan, Thailand) the night before and the morning of the day of surgery. Spinal anesthesia administered was 2.5 mL 0.5% bupivacaine (AstraZeneca, Bangkok, Thailand) and was verified to achieve complete block by a board-certified anesthesiologist. Patients in Group M received spinal anesthesia with 0.2 mg intrathecal morphine sulfate (The Government Pharmaceutical Organization, Bangkok, Thailand) and with periarticular multimodal drug injection in Group I. The multimodal drug injection comprised 100 mg bupivacaine (0.5%, 20 mL), 5 mg morphine sulfate (5 mL), 0.6 mg 1:1000 epinephrine (The Government Pharmaceutical Organization) (0.6 mL), and 30 mg ketorolac tromethamine (Siu Guan Chemical Industrial, Chai Yi, Taiwan) (1 mL). These were mixed with sterile normal saline solution to make up a combined volume of 100 mL. The first 25 mL of mixture was injected into the posterior knee capsule and soft tissue around the medial and lateral collateral ligaments before implantation of the actual components. The quadriceps muscle, retinacular tissues, pes anserinus, and suprapatellar and infrapatellar fat pat then were infiltrated with the rest of the mixture while the cement was curing.
All surgeries were performed by or under the supervision of one surgeon (NT) who was not aware of the anesthetic technique until the bone cut was finished and ready for implantation. A standard medial parapatellar arthrotomy and posterior-stabilized prosthesis were used in all patients. A mobile-bearing knee prosthesis was implanted in patients younger than 60 years. Prostheses used were 51 Genesis II (Smith & Nephew, Memphis, TN, USA) and nine PFC Sigma RPF (DePuy, Johnson & Johnson, Warsaw, IN, USA). The implants were fixed with cement and patellae were not resurfaced. A closed suction drain was placed in the knee capsule before wound closure and removed 48 hours after wound closure. A tourniquet was inflated with pressure equal to the systolic blood pressure of each patient plus 150 mm Hg before skin incision and deflated after wound closure. Tranexamic acid (China Chemical & Pharmaceutical, Hsinchu, Taiwan) 750 mg was injected intravenously 15 minutes before tourniquet was released.
Before surgery, all patients were told how to use the patient-controlled analgesia (PCA) device to control pain aiming for their comfortable level. Postoperatively patients received intravenous PCA for 48 hours using ketorolac. The PCA device was set to inject 0.6 mg in 1 mL (30 mg of ketorolac in 50 mL normal saline) when patients pressed a button with a 2-minute lockout period. There was no continuous infusion and the maximal dose was limited to 15 mg every 4 hours. In addition to PCA, 1300 mg acetaminophen (Janssen Korea, Seoul, Korea) was administered every 8 hours and 10 mg amitriptyline (The Government Pharmaceutical Organization) was administered before bedtime once a day. On the third postoperative day, 250 mg naproxen (Community Pharmacy, Bangkok, Thailand) was administered after meals two times daily and 50 mg tramadol (Stadapharm, Bad Vilbel, Germany) was administered when the patient requested every 6 hours. One 20-mg omeprazole (Berlin Pharmaceutical Industry, Bangkok, Thailand) was given twice daily in all patients since the day of surgery to prevent gastrointestinal bleeding from stress ulcer and NSAIDs. For vomiting we gave 4 mg of ondansetron (Siam Bheasach, Bangkok, Thailand) intravenously every 6 hours until nausea and vomiting improved, and 10 mg chlorpheniramine maleate (The Government Pharmaceutical Organization) was given intravenously every 6 hours for pruritus. The suction drain was removed 48 hours after wound closure in all patients and blood loss collected in the drain was measured.
All patients were encouraged to perform foot pump exercises in bed. All were encouraged to walk for approximately 10 meters with a walker the morning after surgery and they started active and passive ROM exercises by sitting at bedside. All patients were discharged when they met the discharge criteria, which included independent ambulation with a walker for 20 meters, and independently getting in and out of bed. The patients’ postoperative pain was well controlled with oral medications. All patients met discharge criteria and were discharged from the hospital to their homes on postoperative Day 3.
A clinical investigator (SK) who was not aware of the randomization collected demographic data, preoperative clinical conditions using predesigned data sheets, and perioperative data, and entered them in a database. ROM was measured preoperatively and at the 2-, 6-, and 12-week postoperative visits. A modified WOMAC™ score [13] was recorded preoperatively and at postoperative Weeks 6 and 12. Vomiting and pruritus were measured by the number of patients who requested drugs to relieve their symptoms.
Primary outcome variables were the amount of ketorolac consumption through PCA during every 4-hour interval until 48 hours after surgery and pain level which was estimated by the patients using a VAS [23] every 4 hours until 48 hours postoperatively. The VAS for pain ranged from 0 mm (indicating no pain) to 100 mm (indicating extreme pain) in 10-mm increments. Patients were advised to record the VAS for average pain they felt at rest. Secondary outcome variables were morphine-related side effects, blood loss collected in closed suction drainage, ROM, and modified WOMAC score. Morphine-related side effects consisted of the incidence of nausea, vomiting, pruritus, urinary retention, and respiratory depression.
We used a repeated-measures ANOVA to compare the amount of analgesic drug consumption through PCA, VAS for pain, ROM, and modified WOMAC score. Student’s t-test was used to determine differences in blood loss collected in the drain. Fisher’s exact test was used to compare the frequency that patients requested antiemetic or antipruritic drugs. Statistical analyses were conducted using SPSS for Windows (Version 13; SPSS Inc, Chicago, IL, USA).
Results
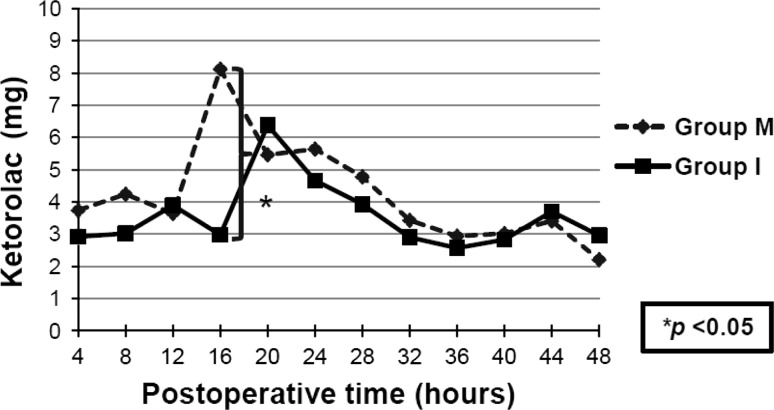
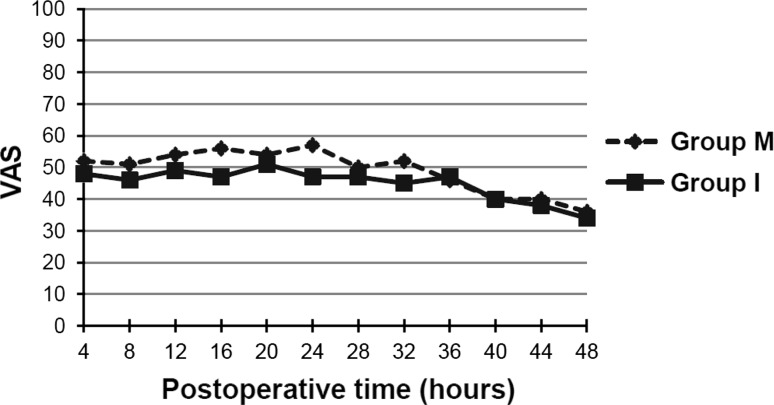
For the first 24 hours after surgery, the average ketorolac consumption was similar (p = 0.22) in the two groups: 30.8 mg in the Group M and 23.8 mg in Group I. We also found no difference (p = 0.34) in overall 48-hour consumption of ketorolac: 50.6 mg versus 42.7 mg in Group M and Group I, respectively. The intrathecal morphine group used more ketorolac (p = 0.02) than the periarticular multimodal drug injection group during the 12- to 16-hour postoperative interval (Fig. 2). The average VAS for pain in both groups which started from approximately 50 during the recovery period decreased to nearly 30 at 48 hours after surgery (Fig. 3). There was no difference in the average VAS for pain (Table 2).
Fig. 2.
The graph shows a comparison of ketorolac consumption postoperatively at every 4-hour interval. Group M = intrathecal morphine; Group I = periarticular multimodal drug injection.
Fig. 3.
The graph shows the VAS for pain every 4 hours until 48 hours postoperatively. Group M = intrathecal morphine; Group I = periarticular multimodal drug injection.
Table 2.
Comparisons of the outcomes between both groups*
| Data | Group M (n = 28) | Group I (n = 29) | p value |
|---|---|---|---|
| Postoperative VAS for pain | |||
| 4 hours | 52 (30) | 48 (31) | 0.58 |
| 8 hours | 51 (28) | 46 (29) | 0.59 |
| 12 hours | 54 (25) | 49 (31) | 0.45 |
| 16 hours | 56 (23) | 47 (28) | 0.21 |
| 20 hours | 54 (20) | 51 (29) | 0.63 |
| 24 hours | 57 (24) | 47 (28) | 0.15 |
| 28 hours | 50 (18) | 47 (28) | 0.60 |
| 32 hours | 52 (23) | 45 (30) | 0.36 |
| 36 hours | 46 (23) | 47 (30) | 0.91 |
| 40 hours | 40 (20) | 40 (25) | 0.99 |
| 44 hours | 40 (20) | 38 (26) | 0.82 |
| 48 hours | 36 (22) | 34 (21) | 0.92 |
| Ketorolac consumption (mg) | |||
| At first 24 hours | 30.8 (24.1) | 23.8 (17.8) | 0.22 |
| At second 24 hours | 19.8 (14.9) | 18.9 (12.0) | 0.79 |
| Overall 48 hours | 50.6 (35.7) | 42.7 (25.1) | 0.34 |
| Modified WOMAC score | |||
| Postoperative 6 weeks | 12 (5) | 13 (5) | 0.38 |
| Postoperative 12 weeks | 9 (4) | 9 (4) | 0.81 |
| ROM (Flexion-degrees) | |||
| Postoperative 2 weeks | 121 (7) | 122 (8) | 0.6 |
| Postoperative 6 weeks | 133 (4) | 133 (5) | 0.85 |
| Postoperative 12 weeks | 140 (4) | 139 (4) | 0.3 |
| Blood loss in drain (mL) | 354 (137) | 308 (184) | 0.29 |
| Wound problem | No | No | – |
* Data presented as mean with SD in parentheses except wound problem presented as incidence.
After surgery, the average modified WOMAC™ score and ROM of knees were similar in both groups. Average blood loss collected from closed suction drainage was approximately 350 mL in both groups. We observed no wound problems in any patient in either group. However, the patients in Group I had two times higher incidence (p = 0.017) of nausea or vomiting when compared with patients in Group M (68% versus 34%, respectively) and nearly four times higher incidence (p = 0.029) of pruritus (36% versus 10%). No incidence of urinary retention or respiratory depression was reported in either group (Table 3).
Table 3.
Side effects and number of patients who needed drugs
| Side effects | Group M (n = 28) | Group I (n = 29) | p value |
|---|---|---|---|
| Nausea and vomiting (number) | 19 (68%) | 10 (34%) | 0.017 |
| Pruritus (number) | 10 (36%) | 3 (10%) | 0.029 |
| Urinary retention | 0 | 0 | – |
| Respiratory depression | 0 | 0 | – |
* Data for side effects presented as numbers with their percentage in parentheses.
Discussion
Postoperative pain after TKA is a primary concern to patients and a focus of recent investigations. Among many approaches, the best techniques remain controversial. Intrathecal morphine or periarticular multimodal drug injection are both commonly used and both appear to provide better pain control than placebo, but it is unclear whether one or the other provides better pain control. We therefore asked whether intrathecal morphine or periarticular multimodal drug injection provides better pain control with minimal side effects.
We recognize limitations in our study. First, the interval between surgery and postoperative Day 1 visit varied as much as 6 hours. The surgeon and his team visited all patients the next day at 9 am; patients who were operated on as the first case of the day would be seen 24 hours after surgery, whereas the second case was 21 hours after surgery and the last was 19 hours. We checked and found that randomization helped equalize the sequence of cases in both groups. Second, we are unable to blind the surgeon because it may be unethical to inject normal saline around the knee capsule, and the surgeon realized the allocation after he had finished the bone cut. Since the patients and observers were blinded, we suspect this would not influence the findings.
We found intrathecal morphine and a periarticular anesthetic cocktail injection controlled pain after a TKA. The VAS for pain started from approximately 50 in the perioperative period down to nearly 30 at 48 hours after surgery. All patients were able to walk with a walker the next morning and were discharged to their homes by the third day after surgery.
The consumption of analgesic drugs during the 12-to 16-hour interval, which was recorded at 16 hours, showed that Group I consumed substantially more analgesics than Group M. The analgesic effect of intrathecal morphine may decline after 10 hours, which is similar to what was reported in a previous study [12]. The analgesic effect of multimodal drug injection may last up to 16 hours.
Intrathecal morphine at 0.2 mg was selected in this study because it had good pain control and minimal morphine-related side effects compared with 0.3 mg [12]. Patients with 0.3 mg intrathecal morphine had better pain relief in the first 24 hours after surgery and used less PCA morphine, but there were side effects such as reduced oxygen saturation, pruritus, nausea, and vomiting [6]. Hassett et al. reported that 0.2 mg and 0.3 mg intrathecal morphine provided comparable postoperative analgesia, which was superior to 0.1 mg intrathecal morphine in patients undergoing TKA [12].
Several studies have reported the efficacy and safety of periarticular multimodal drug injections after TKA (Table 4). No complications related to the infiltration of the local anesthetic were observed, and all plasma concentrations of the local anesthetic were below toxic range [25]. Ropivacaine is pharmacokinetically similar to bupivacaine but it has a longer effect and less cardiac and central nervous system toxicity [8, 22]. In our anesthetic cocktail, we used bupivacaine instead of ropivacaine that frequently was used in previous studies [3, 18, 20, 25] because ropivacaine was not available in our country and bupivacaine has been reported as safe and effective for local infiltration [10, 14].
Table 4.
Comparison of randomized controlled trials of intrathecal morphine and periarticular multimodal drug injection
| Study | Number | Followup time | Intervention | Regimen | Average pain score at 24 hours | Average pain score at 48 hours | Average drug consumption at 24 hours | Knee Society Score© | Flexion motion | Adverse effects | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cole et al. [6] | 34 | 24 hours | Spinal morphine vs placebo | 0.3 mg morphine | VAS movement 30 vs 50 |
NA | Morphine 20 mg vs 40 mg |
NA | NA | No difference in PONV & pruritus | Less pain & morphine consumption in first 24 hours after surgery |
| Hassett et al. [12] | 60 | 24 hours | 0.1 vs 0.2 vs 0.3 mg spinal morphine | 0.1, 0.2, 0.3 mg morphine | VAS 14 vs 11 vs 11 |
NA | Morphine 0.1 mg: 15 mg 0.2 mg: 5 mg 0.3 mg: 4 mg |
NA | NA | No differrence in PONV & pruritus | 0.1 mg spinal morphine had more pain & morphine consumption in first 24 hours after surgery |
| Busch et al. [3] | 64 | 3 months | PAI vs placebo | 400 mg ropivacaine, 30 mg ketorolac, 5 mg morphine, 0.6 mg epinephrine | VAS 45 vs 55 |
NA | Morphine 25 mg vs 45 mg |
KSS Preoperative 87 vs 89 Postoperative 12 weeks: 166 vs 161 WOMAC Preoperative 17 vs 16 Postoperative 12 weeks: 9 vs 9 |
NA | NA | Less pain in PACU & 4 hours after surgery; less morphine consumption in first 24 hours after surgery |
| Vendittoli et al. [25] | 42 | 5 days | Multiple dose PAI vs placebo | Intraoperative 275 mg ropivacaine, 30 mg ketorolac, 0.5 mg epinephrine POD1 (IAI) 150 mg ropivacaine |
VAS 20 vs 30 |
VAS 13 vs 18 |
Morphine 29 mg vs 50 mg |
NA | POD5 90° vs 94° |
Less PONV in PAI | Less pain & morphine consumption up to 48 hours after surgery |
| Essving et al. [9] | 50 | 3 months | Multiple dose PAI vs spinal morphine | Intraoperative Ropivacaine 400 mg, ketorolac 30 mg, epinephrine 0.5 mg POD1&2 (IAI) Ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.1 mg vs 0.1 mg spinal morphine |
VAS rest 5 vs 15 VAS flexion 25 vs 52 VAS walking 19 vs 58 |
VAS rest 5 vs 20 VAS flexion 30 vs 59 VAS walking 10 vs 39 |
Morphine 15 mg vs 30 mg |
OKS Preoperative 39 vs 38 Postoperative 12 weeks: 24 vs 24 |
Preoperative 110° vs 110° Postoperative 12 weeks: 100° vs 100° |
No difference in PONV & pruritus | Less pain & morphine consumption up to 48 hours after surgery |
| Current study | 59 | 3 months | PAI vs spinal morphine | 100 mg bupivacaine, 30 mg ketorolac, 5 mg morphine, 0.6 mg epinephrine vs 0.2 mg spinal morphine | VAS 47 vs 57 |
VAS 34 vs 36 |
Ketorolac 24 mg vs 30 mg |
Modified WOMAC Preoperative 43 vs 50 Postoperative 12 weeks: 9 vs 9 |
Preoperative 121° vs 127° Postoperative 12 weeks: 139° vs 140° |
Less PONV & pruritus in PAI | No difference if pain & ketorolac consumption during 48 hours after surgery; less adverse effects in PAI |
PAI = periarticular injection; IAI = intraarticular injection; POD = postoperative day; PONV = postoperative nausea and vomiting; PACU = postanesthetic care unit; KSS = The Knee Society Clinical Rating Score©; WOMAC™ = Western Ontario and McMaster Universities Osteoarthritis Index; OKS = Oxford Knee Score.
Results from our study indicated that intrathecal morphine had a higher incidence of nausea, vomiting, and pruritus compared with periarticular multimodal drug injection.
PCA ketorolac used in this study helped reduce the confounding effects of morphine used postoperatively and it is reportedly effective for postoperative pain control [4, 17]; 30 milligrams of ketorolac is as effective as 12 mg morphine [2]. In the current study, 48-hour ketorolac consumption in both groups was low (50.6 mg and 42.7 mg). No side effects such as gastrointestinal bleeding were observed. However, we prescribed a proton pump inhibitor (omeprazole) to all patients undergoing TKA to prevent a stress ulcer.
Blood loss collected in a suction drain removed 48 hours after surgery was approximately 350 mL in both groups, which was low compared with loss reported in previous studies [1, 5]. A low-pressure tourniquet at the patients’ systolic blood pressure plus 150 mm Hg, electrocautery used to stop bleeding, and use of tranexamic acid may be the explanation. Epinephrine in the anesthetic cocktail might have some vasoconstrictive effect, but blood collected in the suction drain was not different between the two groups. A larger randomized trial may be needed to further investigate this issue.
Intrathecal morphine and periarticular multimodal drug injection controls pain better than a placebo [3, 6, 25]. We found both techniques provide similar pain control capacity using 0.2 mg intrathecal morphine and a single periarticular injection, but patients who had intrathecal morphine had a higher incidence of nausea, vomiting, and pruritus.
Acknowledgments
We thank all participants for providing the data used in this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Thammasat University, Pathumthani, Thailand.
References
- 1.Aksoy Y, Altinel L, Kose KC. The comparison of the effects of intraoperative bleeding control and postoperative drain clamping methods on the postoperative blood loss and the need for transfusion following total knee arthroplasty. Acta Orthop Traumatol Turc. 2011;45:190–194. doi: 10.3944/AOTT.2011.2398. [DOI] [PubMed] [Google Scholar]
- 2.Brown CR, Mazzulla JP, Mok MS, Nussdorf RT, Rubin PD, Schwesinger WH. Comparison of repeat doses of intramuscular ketorolac tromethamine and morphine sulfate for analgesia after major surgery. Pharmacotherapy. 1990;10:45S–50S. [PubMed] [Google Scholar]
- 3.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88:959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 4.Cepeda MS, Vargas L, Ortegon G, Sanchez MA, Carr DB. Comparative analgesic efficacy of patient-controlled analgesia with ketorolac versus morphine after elective intraabdominal operations. Anesth Analg. 1995;80:1150–1153. doi: 10.1097/00000539-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469:2874–2880. doi: 10.1007/s11999-011-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole PJ, Craske DA, Wheatley RG. Efficacy and respiratory effects of low-dose spinal morphine for postoperative analgesia following knee arthroplasty. Br J Anaesth. 2000;85:233–237. doi: 10.1093/bja/85.2.233. [DOI] [PubMed] [Google Scholar]
- 7.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86:102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Dony P, Dewinde V, Vanderick B, Cuignet O, Gautier P, Legrand E, Lavand’homme P, De Kock M. The comparative toxicity of ropivacaine and bupivacaine at equipotent doses in rats. Anesth Analg. 2000;91:1489–1492. doi: 10.1097/00000539-200012000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Essving P, Axelsson K, Aberg E, Spannar H, Gupta A, Lundin A. Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: a randomized controlled trial. Anesth Analg. 2011;113:926–933. doi: 10.1213/ANE.0b013e3182288deb. [DOI] [PubMed] [Google Scholar]
- 10.Fu P, Wu Y, Wu H, Li X, Qian Q, Zhu Y. Efficacy of intra-articular cocktail analgesic injection in total knee arthroplasty: a randomized controlled trial. Knee. 2009;16:280–284. doi: 10.1016/j.knee.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Cardero P, Rodriguez-Merchan EC. Postoperative analgesia in TKA: ropivacaine continuous intraarticular infusion. Clin Orthop Relat Res. 2010;468:1242–1247. doi: 10.1007/s11999-009-1202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassett P, Ansari B, Gnanamoorthy P, Kinirons B, Laffey JG. Determination of the efficacy and side-effect profile of lower doses of intrathecal morphine in patients undergoing total knee arthroplasty. BMC Anesthesiol. 2008;8:5. doi: 10.1186/1471-2253-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuptniratsaikul V, Rattanachaiyanont M. Validation of a modified Thai version of the Western Ontario and McMaster (WOMAC) osteoarthritis index for knee osteoarthritis. Clin Rheumatol. 2007;26:1641–1645. doi: 10.1007/s10067-007-0560-y. [DOI] [PubMed] [Google Scholar]
- 14.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–130. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 15.Maheshwari AV, Blum YC, Shekhar L, Ranawat AS, Ranawat CS. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res. 2009;467:1418–1423. doi: 10.1007/s11999-009-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahoney OM, Noble PC, Davidson J, Tullos HS. The effect of continuous epidural analgesia on postoperative pain, rehabilitation, and duration of hospitalization in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:30–37. [PubMed] [Google Scholar]
- 17.Park SY, Moon SH, Park MS, Oh KS, Lee HM. The effects of ketorolac injected via patient controlled analgesia postoperatively on spinal fusion. Yonsei Med J. 2005;46:245–251. doi: 10.3349/ymj.2005.46.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6 suppl 2):33–38. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, Murthy Y. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–1162. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 20.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12–15. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen S, Kramhoft MU, Sperling KP, Pedersen JH. Increased flexion and reduced hospital stay with continuous intraarticular morphine and ropivacaine after primary total knee replacement: open intervention study of efficacy and safety in 154 patients. Acta Orthop Scand. 2004;75:606–609. doi: 10.1080/00016470410001501. [DOI] [PubMed] [Google Scholar]
- 22.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–569. [PubMed] [Google Scholar]
- 23.Svensson I, Sjostrom B, Haljamae H. Assessment of pain experiences after elective surgery. J Pain Symptom Manage. 2000;20:193–201. doi: 10.1016/S0885-3924(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 24.Szczukowski MJ, Jr, Hines JA, Snell JA, Sisca TS. Femoral nerve block for total knee arthroplasty patients: a method to control postoperative pain. J Arthroplasty. 2004;19:720–725. doi: 10.1016/j.arth.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 25.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]