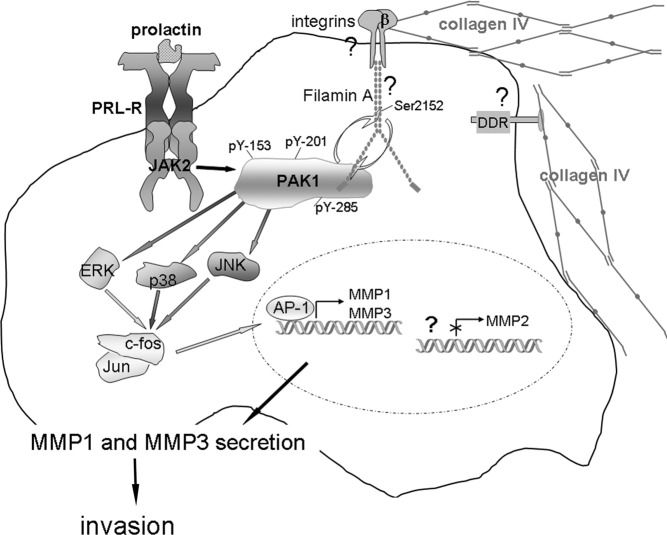
Figure 8.
PRL-Dependent Tyrosyl Phosphorylated PAK1 and 3D Collagen IV Regulate MMP-1 and MMP-3 Production and Invasion via MAPK Pathways Schematic representation of the proposed working model. PRL activation of JAK2 leads to tyrosyl phosphorylation of PAK1 on tyrosines 153, 201, and 285, thereby increasing PAK1 activities (both the serine/threonine kinase activity and ability to create potential protein-protein interactions through these phosphorylates tyrosines) and stimulating phosphorylation of FLNa. Phosphorylated FLNa stimulates the kinase activity of PAK1 in a positive feedback. In turn, FLNa binds to β-integrin and transduces signals from surrounding matrix to inside of a cell. 3D collagen IV-induced signals, in combination with pTyr-PAK1, produce intense synergistic increases in MMP-1 and MMP-3 production via MAPK pathways. MMP-1 degrades type I collagen, which is a major component of the ECM, and MMP-3 degrades collagen IV, which is a main component of basement membrane, resulting in increased invasion of breast cancer cells in response to PRL. NFκB pathway is not shown.