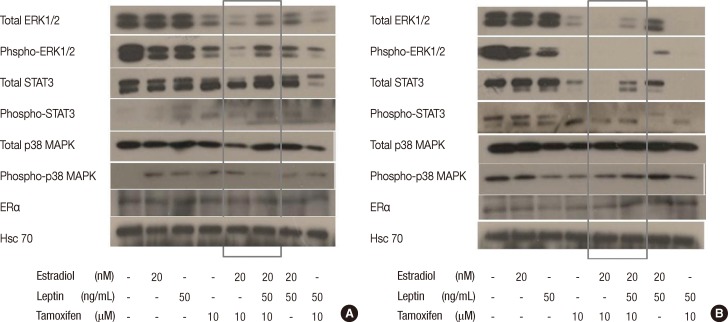
Figure 4.
Leptin activates multiple signal transduction pathways in MCF-7 cells compared to T-47D cells. MCF-7 and T-47D cells were synchronized in serum-free medium and then stimulated with estrogen (20 nM), leptin (50 ng/mL), and tamoxifen (10 µM). Stimulation of Ob-Rb was assessed at different time points from 5 minutes to 6 hours. Hsc70 was used as a loading control at each time point for both cell lines. Activation (phospho) and levels of ERK1/2, STAT3, p38 MAPK, and estrogen receptor α (ERα) were assessed by Western blotting in 25 µg of protein using specific antibodies. Figure 4 shows multiple signal transduction pathways being activated at 1 hour. Tamoxifen 10 µM effectively inhibited the activity of total- and phospho-ERK1/2 and STAT3 in both cell lines. (A) In MCF-7 cells, when leptin was added to the combination of estrogen and tamoxifen, leptin induced activation of total- and phospho-ERK1/2, total- and phospho-STAT3 signaling (grey box). There were no significant differences in total- and phospho-p38 MAPK, and ERα signaling when leptin was added to the combination of estrogen and tamoxifen. (B) In contrast to the results obtained in MCF-7 cells, in T-47D cells, there was no activation of the phospho-ERK1/2 and phospho-STAT3 signal transduction pathways. Also, total- and phospho-p38 MAPK, ERα signaling were not activated by addition of leptin (grey box).