Abstract
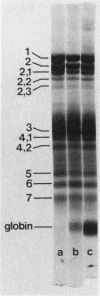
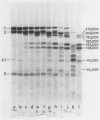
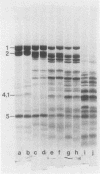
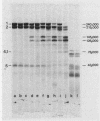
Spectrin, either in the form of unfractionated low ionic strength extracts of erythrocyte membranes or purified by chromatography on Sepharose (CL)4B, was subjected to tryptic digestion at 0 degrees C. Four patients, each with a different variant of hereditary elliptocytosis, were studied. In one patient, whose erythrocytes showed significant fragmentation on heating on 45 degrees C, such preparations generated a remarkably different pattern of polypeptide fragments on tryptic digestion at low ionic strength. In this patient 32P was released at a slower rate on tryptic digestion of labeled band 2, and an unusual 32P-labeled peptide fragment was also generated, in contrast to control preparations in which such a peptide could not be easily distinguished. There was increased susceptibility of this patient's spectrin to tryptic digestion at physiological ionic strength, but the qualitative pattern of polypeptide fragments was normal. Phosphorylation of spectrin by membrane protein kinase was markedly impaired in this patient, whereas phosphorylation of casein ws unimpaired. However, the phosphorylation of spectrin in her intact erythrocytes was normal. Our findings suggest an abnormality of spectrin structure which we postulate is causally related to the predisposition to hemolysis in this patient, but do not distinguish whether this is a primary abnormality or a post-translational modification of the spectrin molecule. The other three patients showed normal tryptic digestion of spectrin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Structural studies on human spectrin. Comparison of subunits and fragmentation of native spectrin. J Biol Chem. 1979 Feb 10;254(3):939–944. [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Beutler E., Guinto E., Johnson C. Human red cells protein kinase in normal subjects and patients with hereditary spherocytosis, sickle cell disease, and autoimmune hemolytic anemia. Blood. 1976 Dec;48(6):887–898. [PubMed] [Google Scholar]
- Calvert R., Ungewickell E., Gratzer W. A conformational study of human spectrin. Eur J Biochem. 1980 Jun;107(2):363–367. doi: 10.1111/j.1432-1033.1980.tb06037.x. [DOI] [PubMed] [Google Scholar]
- Chang K., Williamson J. R., Zarkowsky H. S. Effect of heat on the circular dichroism of spectrin in hereditary pyropoikilocytosis. J Clin Invest. 1979 Jul;64(1):326–328. doi: 10.1172/JCI109456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Erythrocyte membrane proteins in hereditary glucosephosphate isomerase deficiency. J Clin Invest. 1979 Apr;63(4):552–561. doi: 10.1172/JCI109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gomperts E. D., Cayannis F., Metz J., Zail S. S. A red cell membrane protein abnormality in hereditary elliptocytosis. Br J Haematol. 1973 Oct;25(4):415–420. doi: 10.1111/j.1365-2141.1973.tb01753.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Lux S. E., Wolfe L. C. Inherited disorders of the red cell membrane skeleton. Pediatr Clin North Am. 1980 May;27(2):463–486. doi: 10.1016/s0031-3955(16)33862-7. [DOI] [PubMed] [Google Scholar]
- Sheehy R., Ralston G. B. Abnormal binding of spectrin to the membrane of erythrocytes in some cases of hereditary spherocytosis. Blut. 1978 Mar 15;36(3):145–148. doi: 10.1007/BF00996653. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkowsky H. S., Mohandas N., Speaker C. B., Shohet S. B. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975 Apr;29(4):537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]