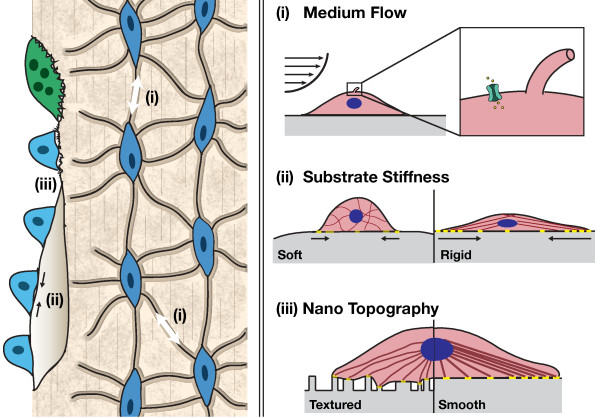
Figure 1.
Role of mechanical cues on osteogenic differentiation. Left: native bone. Physiological loading of bone induces fluid flow within the canaliculi, resulting in shear stress on the osteocytes (i), which transmit these signals to osteoclasts and osteoblasts to remodel the bone. Stiffness (ii) and topography (iii) of native bone matrix also impacts new bone deposition by osteoblastic cells. Right: tissue engineers apply mechanical stimuli to enhance the osteogenic response of stem and progenitor cells in vitro. (i) Perfusion of culture medium over cell monolayers or through three-dimensional constructs imparts shear stress. Shear induces stretching of the cell membrane, allowing an influx of calcium ions through stretch-activated ion channels. Fluid flow also deflects the primary cilia that extend from the surfaces of osteocytes and osteoblasts, altering signal transduction as a result of microtubule tension. (ii) Substrate rigidity influences cell adhesion, spreading, and differentiation patterns. Soft surfaces provide low resistance, decreased focal adhesion (yellow) strength and reduced cytoskeletal organization relative to more rigid surfaces. This leads to changes in nuclear shape and gene expression. (iii) Topography: surface roughness along with spacing and randomness in nanoscale topographical features influence cell adhesion and the formation of localized stresses along the cell membrane. For example, differences in interfeature z-scale dimensions greater than 50 nm impair local focal adhesion strength. These differences are transmitted to the nucleus via actin filaments and lead to changes in gene expression and cell fate.