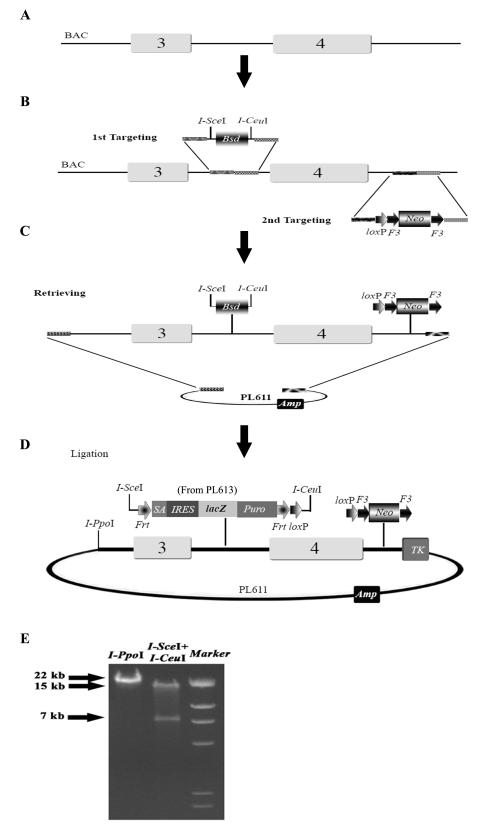
Fig. 1.
The workflow of generating a reporter conditional null targeting vector. (A) The BAC clone containing the region of interest is made recombineering competent by transformed with pSim18 plasmid. Exon 4, which encodes the main functional domains of Bcl11a protein, is the intended deletion region and would be flanked by loxP sites in the conditional allele. (B) The Bsd cassette flanked by two rare cutter sites, I-SceI and I-CeuI, is targeted to intron 3. Subsequently, the loxP-F3-PGK-EM7-Neo-F3 (Neo) cassette is targeted into intron 4. Shaded bars represent the short homology arms (50-80 nts) used for recombineering. In a typical cko vector, we select between 4-5 kb genomic DNA as the left homology arm (5′), and 2-3kb as the right homology arm (3′). The genomic DNA region to be deleted is generally between 1-7 kb. (C) The doubly targeted genomic DNA on the BAC is retrieved to PL611 (AmpR). (D) The Bsd cassette is replaced by the lacZ reporter cassette (from PL613) in a simple restriction digestion and ligation process. The final targeting vector has the lacZ reporter flanked by two FRT sites with one loxP site, and has the F3-flanked Neo cassette with another loxP site. Finally, the negative selection marker MC1TK is added to the vector backbone by recombineering. The final targeting vector is linearized with I-PpoI for ES cell transfection. The true conditional knockout allele is obtained by excising the lacZ and the Neo cassettes with Flpe in the targeted ES cells or in the mouse germline. We have shown that FRT and F3 sites do not recombine in the mouse germline with the constitutive presence of Flpe. (E) Restriction digestion patterns of the final targeting vector. Restriction digestion with I-PpoI linearizes the targeting vector (22 kb). Digestion with both I-SceI and I-CeuI excises the 7 kb lacZ reporter cassette.