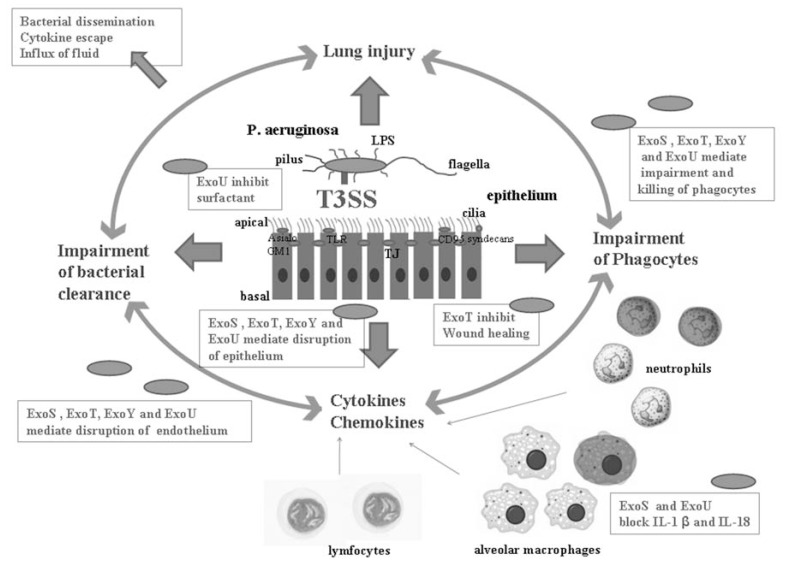
Fig. (5).
Role of the T3SS in pathogenicity and host responses to P. aeruginosa acute lung infection. The T3SS creates a self-propagating pathogenic cycle. T3SS induced acute lung injury is connected with T3SS induced enhancement of proinflammatory mediators, such as cytokines and chemokines, and a decrease in the number of alveolar phagocytes. Both of these lead to impairment of bacterial clearance from the lungs and a higher mortality rate. The darkly stained phagocytes are killed by a T3SS dependent mechanism. The airway epithelial cells, macrophages, neutrophils, and lymphocytes release mediators that enable the host to mount a response to the invading bacteria. The T3SS facilitates tissue injury and invasion of the pathogen by different mechanisms. ExoS, ExoT, ExoY and ExoU induce cell death of epithelial cells, ExoT inhibits wound healing, and ExoU inhibits surfactant production. Together, these lead to disruption of the alveolar epithelium. ExoU and ExoS can block the production of IL-1β and IL-18 induced by the T3SS needle and diminish the early inflammatory response. However, the inflammatory response is often excessive and induces lung injury. ExoS, ExoT, ExoY and ExoU mediate the impairment and killing of phagocytes, which impairs bacterial clearance. ExoS, ExoT, ExoY and ExoU mediate the disruption of both the epithelial and the endothelial barriers, which leads to dissemination of the pathogen, escape of cytokines into the systemic circulation, and influx of protein rich fluids. The consequence is septic bacteremia and shock.