Abstract
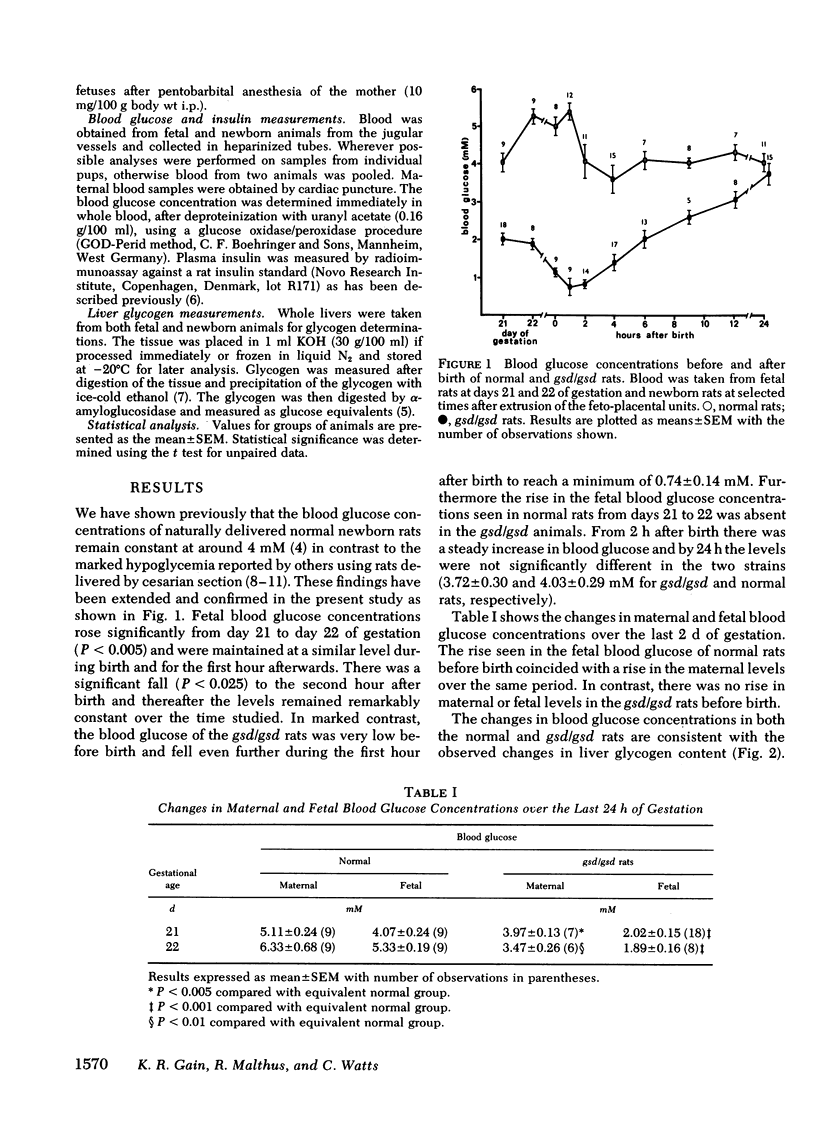
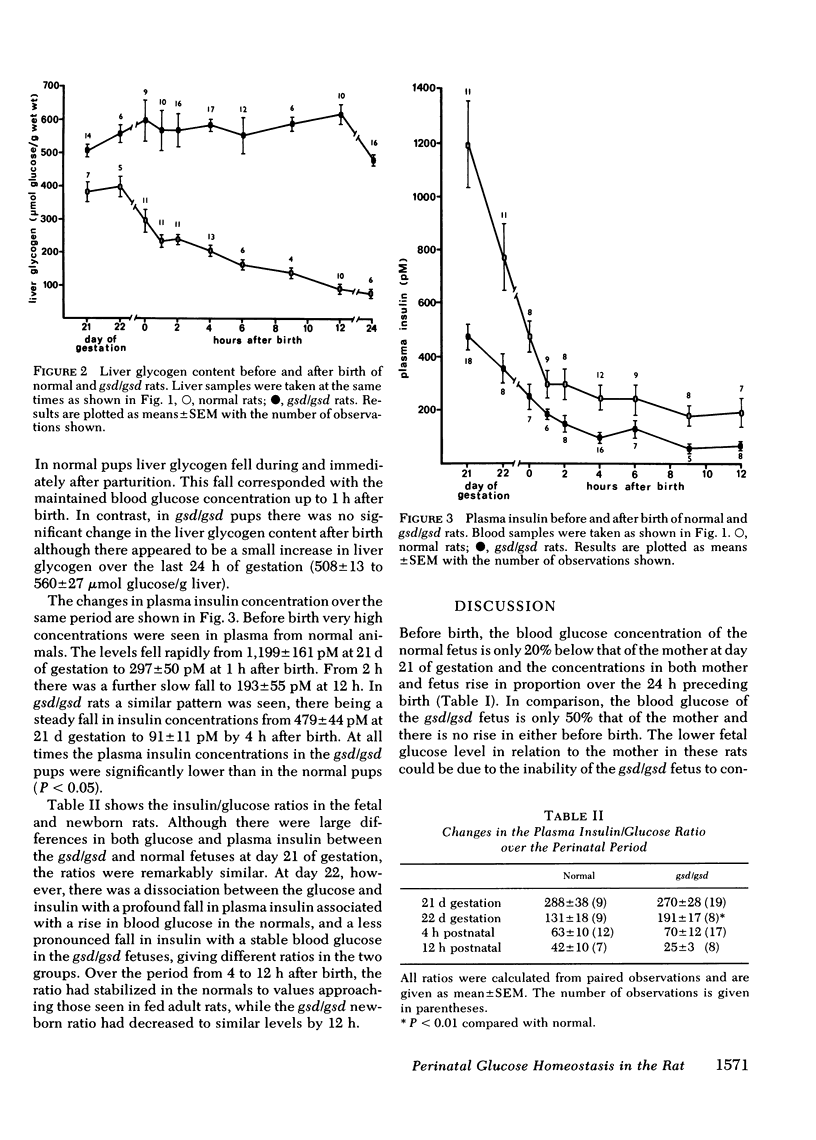
The fetal rat mobilizes liver glycogen during parturition for use as a glucose source until the onset of gluconeogenesis at 2 h after birth. A rat strain (NZR/Mh) unable to mobilize liver glycogen because of a phosphorylase b kinase deficiency has been used to assess the importance of liver glycogen in glucose homeostasis of the newborn. In normal rats the mean blood glucose concentration of the fetus measured at various times up to 24 h after natural birth ranged between 3.7 and 5.4 mM. In contrast, fetuses of the affected rats were hypoglycemic before birth (2.02 +/- 0.15 mM), and by 1 h after birth the blood glucose had decreased to 0.74 +/- 0.14 mM. Concentrations increased by 4 h to 1.48 +/- 0.17 mM and by 24 h reached values not significantly different from the normal newborn rats. Changes in plasma insulin over the perinatal period were similar in both groups although concentrations were always significantly lower in the affected rts. The findings demonstrate the crucial role of the fetal liver glycogen store in the maintenance of normoglycemia in the newborn. The normal rat does not develop hypoglycemia when born naturally and left with the mother after birth (in contrast to other studies in which the newborn were taken by cesarian delivery 1 d prematurely and kept in an artificial environment without food). The rats with the glycogen storage disorder experienced severe hypoglycemia without any apparent effects, which raises questions concerning alternative fuels available to and utilized by the newborn.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J. The development of gluconeogenesis in rat liver. Controlling factors in the newborn. Biochem J. 1971 Sep;124(2):265–274. doi: 10.1042/bj1240265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cake M. H., Oliver I. T. The activation of phosphorylase in neonatal rat liver. Eur J Biochem. 1969 Dec;11(3):576–581. doi: 10.1111/j.1432-1033.1969.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Cohen N. M., Turner R. C. Plasma insulin in the foetal rat. Biol Neonate. 1972;21(1):107–111. doi: 10.1159/000240500. [DOI] [PubMed] [Google Scholar]
- Devos P., Hers H. G. Glycogen metabolism in the liver of the foetal rat. Biochem J. 1974 May;140(2):331–340. doi: 10.1042/bj1400331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix J. M., Sutter M. T., Sutter B. C., Jacquot R. Circulating insulin and tissular reactivity to insulin in the rat during the perinatal period. Horm Metab Res. 1971 Mar;3(2):71–75. doi: 10.1055/s-0028-1095030. [DOI] [PubMed] [Google Scholar]
- Freinkel N. The role of nutrition in medicine. Recent developments in fuel metabolism. JAMA. 1978 May 5;239(18):1868–1872. [PubMed] [Google Scholar]
- Gilbert M., Bourbon J. Turnover of liver glycogen in the rat foetus. Biochem J. 1978 Dec 15;176(3):785–789. doi: 10.1042/bj1760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Cuendet G. S., Marliss E. B., Kervran A., Rieutort M., Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973 Dec;52(12):3190–3200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S., Russell J. C., Taylor A. W. Determination of glycogen in small tissue samples. J Appl Physiol. 1970 Feb;28(2):234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- Malthus R., Clark D. G., Watts C., Sneyd J. G. Glycogen-storage disease in rats, a genetically determined deficiency of liver phosphorylase kinase. Biochem J. 1980 Apr 15;188(1):99–106. doi: 10.1042/bj1880099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce P. H., Buirchell B. J., Weaver P. K., Oliver I. T. The development of phosphopyruvate carboxylase and gluconeogenesis in neonatal rats. Biol Neonate. 1974;24(5):320–329. doi: 10.1159/000240664. [DOI] [PubMed] [Google Scholar]
- Philippidis H., Ballard F. J. The development of gluconeogenesis in rat liver: experiments in vivo. Biochem J. 1969 Jul;113(4):651–657. doi: 10.1042/bj1130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Rall T. W. Hormonal regulation of glycogen metabolism in neonatal rat liver. Biochem J. 1973 Aug;134(4):985–993. doi: 10.1042/bj1340985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh G. E., Mrozak S. C., Freinkel N. Fetal fuels. I. Utilization of ketones by isolated tissues at various stages of maturation and maternal nutrition during late gestation. Metabolism. 1977 Jun;26(6):623–635. doi: 10.1016/0026-0495(77)90084-1. [DOI] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Glucose metabolism in the newborn rat. Temporal studies in vivo. Biochem J. 1973 Apr;132(4):739–752. doi: 10.1042/bj1320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Glucose metabolism in the newborn rat: the role of insulin. Diabetologia. 1978 Jan 14;14(1):59–64. doi: 10.1007/BF00429709. [DOI] [PubMed] [Google Scholar]
- Sodoyez-Goffaux F. R., Sodoyez J. C., De Vos C. J. Insulin secretion and metabolism during the perinatal period in the rat. Evidence for a placental role in fetal hyperinsulinemia. J Clin Invest. 1979 Jun;63(6):1095–1102. doi: 10.1172/JCI109401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDOWSON E. M. Chemical composition of newly born mammals. Nature. 1950 Oct 14;166(4224):626–628. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- Watts C., Gain E. R. Glycogen metabolism in the liver of the developing rat. Biochem J. 1976 Nov 15;160(2):263–270. doi: 10.1042/bj1600263. [DOI] [PMC free article] [PubMed] [Google Scholar]


