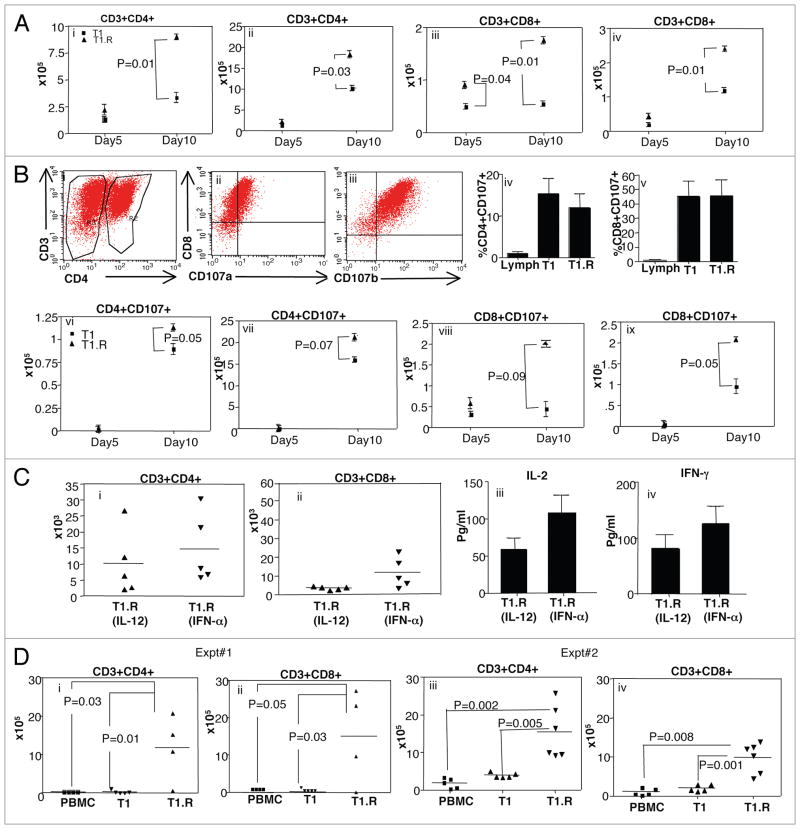
Figure 10.
T1.R cells have increased persistence in vivo. (A) T1 and T1.R cells were generated and adoptively transferred into immune-deficient murine hosts. At day 5 and day 10 after adoptive transfer, the absolute number of human CD4+ T cells were enumerated in the spleen (part i) and bone marrow (part ii); similarly, the absolute number of human CD8+ T cells were enumerated in the spleen (part iii) and bone marrow (part iv; n = 10 per group). (B) The cytolytic function of T1 and T1.R cells was evaluated by flow cytometric expression of the degranulation marker, CD107 (representative results, parts i–iii). Summation of results is shown in (parts iv and v) (n = 10 per group); “Lymph” indicates results using unmanipulated human T cells. After adoptive transfer the absolute number of CD4+CD107+ and CD8+CD107+ cells in spleen and bone marrow at day5 and day 10 post-infusion were enumerated (parts vi–ix). (C) Human T1.R cells were generated by either priming in IL-12 (“T1.R [IL-12]”) or IFNα (“T1.R[IFNα]”) and injected into Rag2−/−γc−/− murine hosts (n = 5 per cohort). (C) At day 30 post-transplant, the absolute number of human CD3+CD4+ T cells (part i) and CD3+CD8+ T cells (part ii) in the spleen was determined by flow cytometry and cell counting. Also at day 30, serum from transplant recipients was evaluated for IL-2 (part iii) and IFNγ (part iv) content by Luminex® assay. (D) In a separate transplant experiment, murine host received either unmanipulated peripheral blood mono-nuclear cells (“PBMC”), control Th1/Tc1 cells generated by co-stimulation in the presence of IL12 (“T1”), or RR-Th1/Tc1 cells generated by costimulation in the presence of both IL12 and rapamycin (“T1.R”). At day 48 post-transplant, the absolute number of human CD3+CD4+ T cells (part i) and CD3+CD8+ T cells (part ii) in the spleen was determined by flow cytometry and cell counting. (Parts iii and iv) show engraftment results from a second experiment.