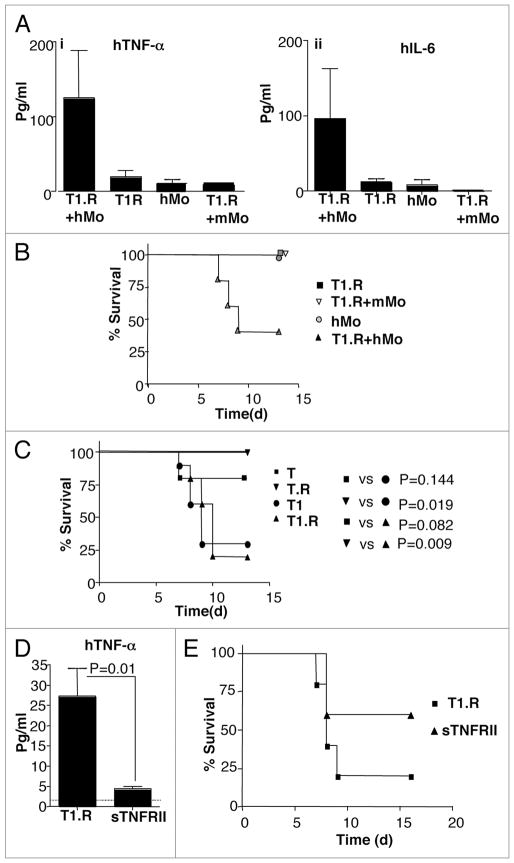
Figure 9.
Type I cytokine polarized co-stimulated human T cells mediate increased LPS-induced, TNFα mediated lethal xenogeneic GVHD. Human Th1/Tc1 cells were generated by co-stimulation ± type I cytokine priming with IFNα (1 M IU/ml) and ±rapamycin to generate four distinct human T cell populations denoted as “T” (no IFNα, no rapamycin), “T.R” (no IFNα, +rapamycin), “T1” (+IFNα, no rapamycin), and “T1.R” (+IFNα, +rapamycin). (A) A pilot experiment was performed to confirm the role of human monocytes for induction of cytokine-mediated GVHD. Rag2−/−γc−/− murine hosts received some combination of human T1.R cells, human monocytes (“hMo”), or murine monocytes (“mMo”), as indicated. On day 5 after adoptive transfer, mice received bacterial LPS; at 90 min post-LPS, serum was obtained and evaluated for human TNFα (part i) and IL-6 (part ii) content by Luminex® assay (results pooled from n = 5 recipients per cohort). (B) Cohorts of mice in this same experiment were also evaluated for post-LPS lethality (n = 5 per cohort). (C) In a separate experiment, Rag2−/−γc−/− murine hosts received human monocytes plus one of the four separate human T cell populations (T, T.R, T1 or T1.R), with subsequent assessment of post-LPS lethality (n = 10 subjects per cohort). (D) To evaluate whether lethality in this model was TNFα mediated, murine host received human monocytes and T1.R cells either alone (“T1.R”) or with in vivo injection of soluble TNF receptor type II (“T1.R + sTNFRII”). On day 5 post-transplant, LPS was injected, and the 90 min post-LPS serum was evaluated for content of human TNFα by Luminex® assay (results pooled from n = 5 recipients per cohort). (E) Cohorts of mice in the same experiment were also evaluated for post-LPS lethality (n = 10 recipients per cohort).