Abstract
Background
The extent to which anemia management is facilitated by more frequent hemodialysis (HD) is controversial. We hypothesized as a preselected outcome that patients receiving HD six times (6×) compared with three times (3×) per week would require lower doses of erythropoietin-stimulating agents (ESA) and/or achieve higher blood hemoglobin (Hb) concentrations.
Methods
Subjects enrolled in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials were studied. As the primary outcome for anemia, the dose of ESAs was recorded at 4-month intervals and the monthly dose of intravenous iron (IV Fe) was reported. Serum iron, transferrin saturation and ferritin were measured at baseline and then at 4-month intervals, whereas Hb concentration was measured monthly.
Results
There was no significant treatment effect in the 6× versus 3× treatment groups on logESA dose or the ratio of log of ESA dose to Hb concentration in either trial. In the daily trial, Hb concentrations increased significantly in the 6× versus 3× group, at Month 12 compared with baseline (0.3 g/dL; 95% CI: 0.05–0.58, P < 0.021), but both groups had Hb concentrations in the usual target range. In the daily trial, the weekly logESA dose and the logESA dose to Hb concentration ratio tended to decline more in the 6× versus 3× group. This trend was not observed in the nocturnal trial. IV Fe doses were significantly lower in the 6× compared with the 3× group by Month 12 in the nocturnal trial, but not different in the daily trial.
Conclusions
In the FHN Daily and Nocturnal Trials, more frequent HD did not have a significant or clinically important effect on anemia management.
Keywords: anemia, erythropoietin, frequent, hemodialysis, nocturnal
INTRODUCTION
The management of anemia remains a controversial and costly problem for patients on maintenance hemodialysis (HD) [1]. A dialysis strategy that would improve hemoglobin (Hb) concentration, while lowering the dose requirements of erythropoietin-stimulating agents (ESAs) would be an important benefit. Previous reports on the impact of frequent HD treatments on Hb levels and ESA dose have demonstrated inconsistent results [2–5]. More frequent HD might reduce ESA requirements by improving the uremic environment, reducing inflammation and thereby lowering ESA resistance [6]. Red blood cell survival might increase toward normal when the uremic toxin concentrations are lowered by more frequent HD or survival might decrease when more frequent HD treatments damage red blood cells in the extracorporeal circuit [7]. Blood loss due to residual blood remaining in the dialyzer at the end of each treatment might increase overall blood loss with more frequent treatments [5, 8].
The Frequent Hemodialysis Network trials (FHN) were designed to explore whether frequent HD administered during the day, in-center, with relatively short session lengths, or at night, at home using relatively longer session length, would improve left ventricular hypertrophy and health-related quality of life [9]. The primary results of both the FHN Daily Trial [10] and the FHN Nocturnal Trial [11] have been reported. Anemia management was preselected as one of nine other secondary outcomes. This report describes the effects of more frequent HD in the daily and nocturnal trials on measures related to anemia management. The main prespecified outcome measure was the change in equivalent dose of ESA from baseline to the end of the trial.
MATERIALS AND METHODS
The FHN trials were prospective, randomized, multicenter studies of HD delivered six times per week (6×) compared with three times per week (3×). Details of the study design and primary outcomes have been published previously [9–11]. The studies were approved by Institutional Review Boards at participating sites and were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Studies were registered at ClinicalTrials.gov (daily: NCT00264758; nocturnal: NCT00271999). An independent data and Safety Monitoring Board reviewed safety data and interim results.
The dose and route of administered ESAs was reported as total units administered over 4-week period (at baseline and Months 4, 8 and 12). This dose was then divided by four for expression as a total, weekly average dose. Dose was calculated in terms of equivalent dose of erythropoietin; when darbepoetin was used, the equivalent dose of erythropoietin was calculated using a standard conversion [12]. In subjects receiving intravenous iron (IV Fe), the total dose given for each month was recorded. The prescribed dose of ESA and IV Fe was left to each patient's individual nephrologist. The FHN protocol contained standards of care which for anemia used the KDOQI guidelines from 2006 [13]. Primary nephrologists were encouraged to use these standard guidelines and reports were reviewed periodically by a standard of care committee. Clinical centers were specifically notified when patients were out the specified range (Hb concentration <10 or >12 g/dL).
Outcome measures
The change in dose of ESA from baseline to Month 12 was the primary, preselected outcome measure for the anemia domain. To further, study the impact of more frequent HD, additional comparisons were made such as Hb concentrations (see section ‘Statistical analysis’).
Laboratory measurements
Hb concentrations were obtained predialysis, at baseline and monthly throughout the follow-up. Serum iron, transferrin saturation, total iron-binding capacity and ferritin were measured at baseline and at 4-month intervals during the follow-up. These laboratory values were all measured at local laboratories with results transmitted to a central data coordinating center.
Statistical analysis
Subjects who died or received a renal transplant were censored at the time of these events. Subjects were also censored if lost to the follow-up. Treatment comparisons between 6× and 3× arms in each trial were made based on change in ESA dose from baseline to Month 12 using mixed-effects models [14]. These models were used to allow comparison at other time points as well. Because of the large variation in ESA and iron doses administered, log transformations were applied and adjusted Windsorized geometric means were utilized for comparisons [15]. The ratio of a single dose of ESA transformed to logESA divided by the Hb concentration (obtained the same month) was also calculated and used as a surrogate indicator of erythropoietin resistance [16]. The monthly Hb concentrations were plotted and the change in Hb concentration from baseline to the end of the trial was determined. End of trial values for Hb concentration were calculated as the average of levels measured for Months 10 through 12.
In the daily trial, additional analyses focused on whether the effect of more frequent dialysis on the primary outcome, change in ESA dose, was modified in prespecified subgroups. Four such subgroups were studied: (i) patients with shorter (<4 years) versus longer (≥4 years) ESRD vintage (with vintage defined by total years since onset of ESRD), (ii) younger (≤50 years) versus older (>50 years) patients, (iii) those with residual renal function ≤1 versus >1 mL/min and (iv) patients with or without diabetes mellitus. For this comparison, the treatment effect studied was again on change from baseline to Month 12 in log weekly ESA dose. In the nocturnal trial, given the relatively small number of subjects, these analyses were not done.
RESULTS
Between 1 January 2006 and 31 March 2009, 378 patients were enrolled and 245 were randomized in the daily FHN trial. In the nocturnal trial, 118 subjects were enrolled and 87 were randomized. There was equal distribution of censored patients between the treatment arms in both the daily (total censored 29) and the nocturnal trials (total censored 6). The baseline characteristics of patients in both trials are presented in Table 1 and were similar for subjects assigned to the 6 and the 3× groups. More extensive comparisons are reported elsewhere [10, 11]. Subjects in both trials demonstrated substantial adherence to the treatment schedules [10, 11].
Table 1.
Characteristics during baseline for FHN patients
| Variables | Daily trial |
Nocturnal trial |
||
|---|---|---|---|---|
| 3×/week (n = 120) (conventional) | 6×/week (n = 125) (daily) | 3×/week (N = 42) (conventional) | 6×/week (N = 45) (nocturnal) | |
| Age (years) | 52.0 ± 14.1 | 48.9 ± 13.6 | 52.0 ± 14.1 | 48.9 ± 13.6 |
| Male (%) | 73 (60.8) | 78 (62.4) | 73 (60.8) | 78 (62.4) |
| Race/ethnicity (%) | ||||
| Black | 53 (44.2) | 49 (39.2) | 11 (26.2) | 12 (26.7) |
| White | 46 (38.3) | 43 (34.4) | 21 (50.0) | 27 (60.0) |
| Native American, Aboriginal Canadian | 4 (3.3) | 4 (3.2) | 2 (4.8) | 1 (2.2) |
| Alaskan native, first nation (%) | ||||
| Asian | 5 (4.2) | 11 (8.8) | 7 (16.7) | 5 (11.1) |
| Native Hawaiian or other Pacific | 3 (2.5) | 1 (0.8) | 0 (0) | 0 (0) |
| Islander (%) | ||||
| Other/mixed/unknown | 9 (7.5) | 17 (13.6) | 1 (2.4) | 0 (0) |
| Hispanic/Latino ethnicity | 31 (26) | 38 (30) | 0 (0) | 0 (0) |
| ESRD vintage (years) (%) | ||||
| <1 year | 20 (17) | 90 (72) | 11 (26) | 13 (29) |
| 1 to < 2 years | 15 (12) | 21 (17) | 16 (38) | 9 (20) |
| 2–5 years | 42 (35) | 6 (4.8) | 6 (14) | 11 (24) |
| >5 years | 43 (36) | 7 (5.6) | 9 (21) | 12 (27) |
| Weekly standard Kt/V | 2.53 ± 0.39 | 2.50 ± 0.31 | 2.34 ± 0.34 | 2.35 ± 0.28 |
| Residual urine volume (L/24 h) | 0 (0, 0.54) | 0 (0, 0.60) | 1.84 (0, 9.11) | 3.18 (0, 8.63) |
| Residual renal clearance (Kru + KCr)/2) mL/min (%) | ||||
| =0 | 72 (60) | 90 (72) | 11 (26) | 13 (29) |
| >0–2 | 19 (15.8) | 18 (14.4) | 16 (38) | 9 (20) |
| >2–4 | 27 (22.5) | 15 (12.0) | 6 (14) | 11 (24) |
| > 4 | 2 (1.7) | 2 (1.6) | 9 (21) | 12 (27) |
| Hypertension (%) | 111 (92.5) | 117 (93.6) | 3 (7.1) | 4 (8.9) |
| Coronary artery disease MI (%) | 16 (13.3) | 11 (8.8) | 4 (9.5) | 5 (11.1) |
| Congestive heart failure (%) | 24 (20.0) | 25 (20.0) | 7 (16.7) | 5 (11.1) |
| Atrial fibrillation (%) | 9 (7.5) | 5 (4.0) | 0 (0.0) | 6 (13.3) |
| Peripheral arterial disease (%) | 10 (8.33) | 15 (12.0) | 7 (16.7) | 8 (17.8) |
| Stroke (%) | 9 (7.5) | 9 (7.2) | 1 (2.4) | 1 (2.2) |
| Diabetes (%) | 50 (41.7) | 50 (40.0) | 18 (42.9) | 19 (42.2) |
| COPD (%) | 5 (4.2) | 6 (4.8) | 2 (4.8) | 2 (4.4) |
| Liver disease (%) | 1 (0.8) | 1 (0.8) | 1 (2.4) | 0 (0) |
| Dialysis access at randomization (%) | ||||
| Fistula | 71 (59.2) | 82 (65.6) | 17 (40.5) | 22 (48.9) |
| Graft | 23 (19.2) | 22 (17.6) | 4 (9.5) | 3 (6.7) |
| Catheter | 26 (21.7) | 21 (16.8) | 21 (50.0) | 20 (44.4) |
Results are shown as mean ± standard deviation, median and 10 and 90th percentiles range or frequency (%), as appropriate.
ESA dose
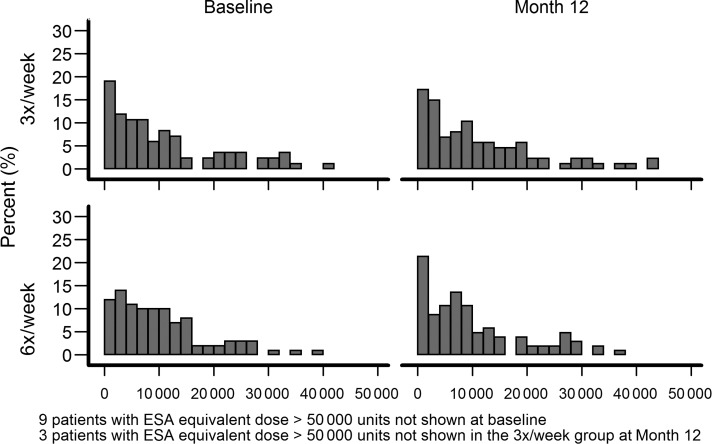
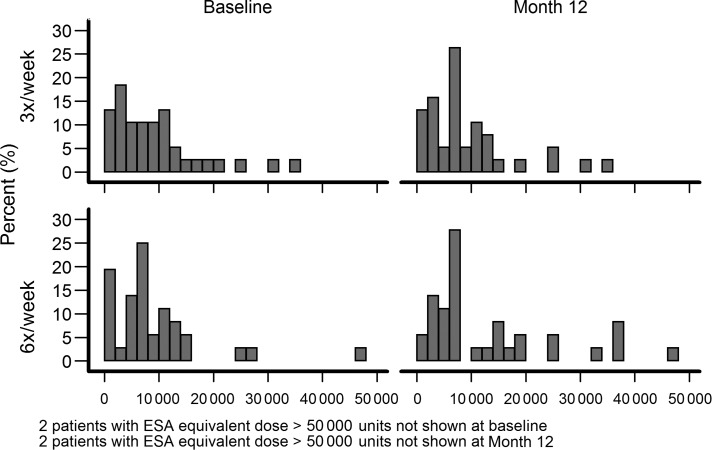
The main predesignated outcome in the anemia domain was the dose of ESA. The range in ESA doses being prescribed for subjects in both trials was quite large as depicted in the histograms in Figures 1 and 2. At baseline, there was no difference in the adjusted Windsorized geometric means of weekly ESA dose between groups in either trial. The route of administration for ESA in both trials, at baseline, was almost universally per IV as opposed to the subcutaneous route; three daily and five nocturnal patients were on subcutaneous ESA. At baseline, 17 patients in the daily trial were not receiving ESAs and by Month 12 this number dropped to 7. In the nocturnal trial, 12 subjects at baseline and 2 at Month 12 were not receiving ESAs.
FIGURE 1:
The percent of patients receiving the wide ranges of weekly ESA doses in the daily FHN trial. Note those subjects receiving >50 000 units/week were not plotted.
FIGURE 2:
The percent of patients receiving the wide ranges of weekly ESA doses in the nocturnal FHN trial. Note those subjects receiving >50 000 units per week were not plotted.
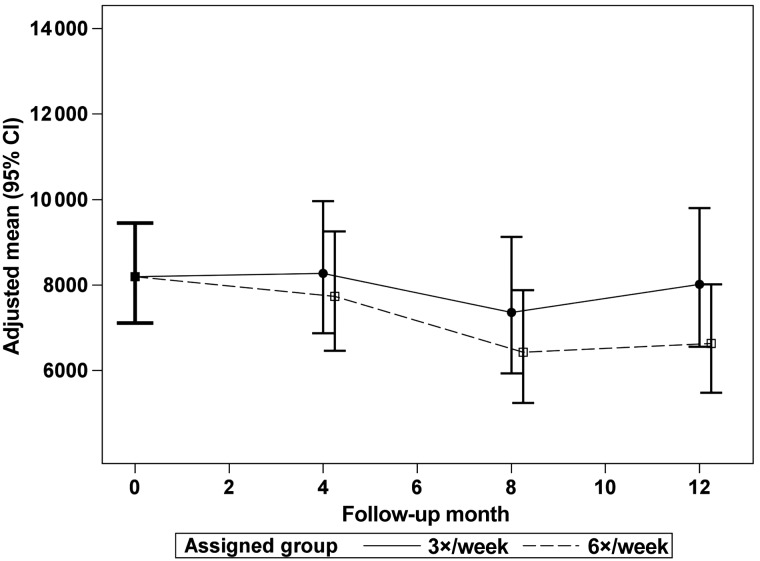
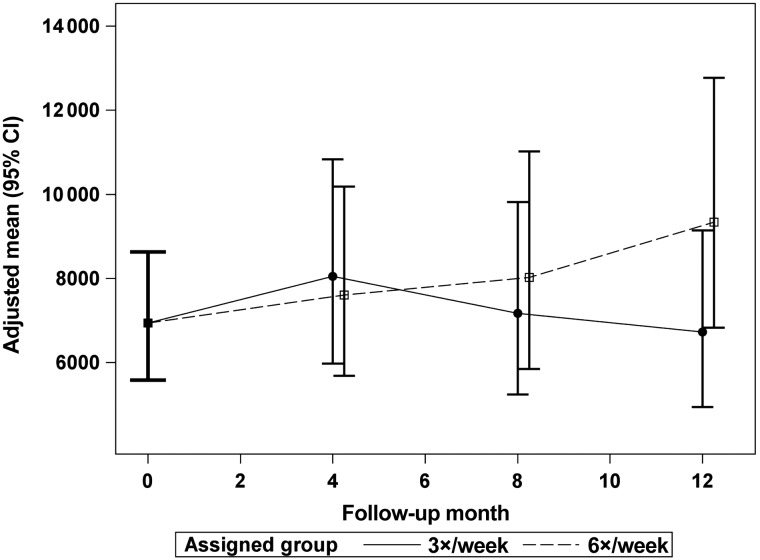
The Windsorized geometric mean ESA doses are plotted from a common mean to the doses at Months 4, 8 and 12 for the 6× and 3× groups in the daily trial (Figure 3) and the nocturnal trial (Figure 4). There was no significant treatment effect of frequent HD on ESA dose in either trial. Table 2 shows the effect of frequent HD on the average weekly dose of ESA, the log of the weekly dose of ESA and the ratio of log transformed weekly ESA dose to Hb concentration ratio at Months 4 and 12 for the daily trial. Table 3 shows the same measures for the nocturnal trial. There was no significant effect of frequent HD on any of these measures.
FIGURE 3:
The adjusted, Windsorized geometric means for weekly ESA dose (equivalent units per week) in the daily trial in the 6× and 3× groups with a common mean at baseline and at follow-up Months 4, 8 and 12. There was no significant difference between the groups at any follow-up time.
FIGURE 4:
The adjusted, Windsorized geometric means for weekly ESA dose (equivalent units per week) in the nocturnal trial in the 6× and 3× groups with a common mean at baseline and at follow-up Months 4, 8 and 12. There was no significant difference between the groups at any follow-up time.
Table 2.
Daily trial
| Outcome | Group | Observed data median (10th, 90th percentiles) |
Adjusted means and treatment effectsa(95% confidence intervals) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | F4 | F12 | Month 4 |
Month 12 |
||||
| Change from baseline | Treatment comparison(6 versus 3×) | Change from baseline | Treatment comparison(6 versus 3×) | |||||
| ESA per weeka | 3× | 8638 (713, 33 125) | 8125 (394, 30 000) | 8925 (563, 31 025) | 0.9 (−14.3, 18.8) | −6.5 (−24.8, 16.3) | −2.3 (−19.8, 19.1) | −17.2 (−35.8, 6.8) |
| 6× | 8500 (1563, 24 000) | 7800 (1000, 27 000) | 7000 (400, 26 000) | −5.7 (−19.4, 10.3) | −19.1 (−32.8, −2.6) | |||
| Predialysis hemoglobin (g/dL) | 3× | 11.9 (10.3, 13.8) | 12.0 (10.9, 13.2) | 11.7 (10.6, 12.8) | 0 (−0.22, 0.23 ) | 0.17 (−0.08, 0.43) | −0.24 (−0.48 ,0.00 ) | 0.33 (0.05, 0.61)* |
| 6× | 12.0 (10.6, 13.3) | 12.2 (11.0, 13.3) | 11.9 (10.9, 13.1) | 0.18 (−0.04 ,0.39 ) | 0.09 (−0.15 ,0.32) | |||
| ESA/hemoglobin ratioa | 3× | 720 (106, 2948) | 691 (113, 2778) | 794 (100, 2783) | 1.46 (−14.09, 19.83) | −8.71 (−26.9, 14.0) | 0.54 (−18.01, 23.3) | −20.06 (−38.6, 4.07) |
| 6× | 743 (123, 2119) | 651 (108, 2375) | 632 (98, 2187) | −7.38 (−20.99, 8.58) | −19.63 (−33.67, −2.62) | |||
| Monthly IV iron (mg)a | 3× | 100 (0, 500) | 100 (0, 300) | 100 (0, 400) | −32.27 (−60.17, 15.15) | −0.11 (−47.87, 91.41) | −28.79 (−59.77, 26.02) | 86.3 (−5.88, 268.76) |
| 6× | 100 (0, 500) | 100 (0, 400) | 100 (0, 400) | −32.35 (−59.81, 13.89) | 32.66 (−23.02, 128.61) | |||
| Predialysis transferrin (%) | 3× | 28 (16, 41) | 29 (15, 44) | 28 (14, 43) | 2.31 (−0.24, 4.85) | −2.19 (−5.46, 1.08) | −1.12 (−3.74, 1.51) | 0.48 (−2.54, 3.5) |
| 6× | 27 (15, 55) | 28 (17, 47) | 28 (17, 43) | 0.12 (−2.36, 2.6) | −0.63 (−3.09, 1.83) | |||
| Predialysis ferritin (ng/mL) | 3× | 572 (208, 947) | 605 (165, 1010) | 617 (176, 1239) | 89.26 (20.77, 157.75) | −57.4 (−151.6, 36.8) | 98.3 (24.0, 172.6) | −98.4 (−197.6, 0.8) |
| 6× | 517 (245, 1129) | 568 (203, 1222) | 554 (190, 1075) | 31.9 (−34.63, 98.44) | −0.1 (−69. 6, 69.3) | |||
a% changes in geometric means for ESA dose, ESA/hemoglobin ratio and monthly IV iron.
*P < 0.05.
Table 3.
Nocturnal trial
| Outcome | Group | Observed data median (10th, 90th percentiles) |
Adjusted means and treatment effectsa (95% confidence intervals) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | F4 | F12 | Month 4 |
Month 12 |
||||
| Change from baseline | Treatment comparison(6 versus 3×) | Change from baseline | Treatment comparison(6 versus 3×) | |||||
| ESA per weeka | 3× | 7750 (0, 25 800) | 11 250 (1563, 25 000) | 7000 (1250, 37 500) | 16.0 (−14.4, 57.1) | −5.46 (−36.1, 39.8) | −3.08 (−31.3, 36.8) | 38.8 (−9.87, 113.9) |
| 6× | 6563 (0, 25 800) | 10 000 (0, 25 000) | 6250 (2250, 37 500) | 9.65 (−18.60, 47.70) | 34.6 (−5.13, 90.9) | |||
| Predialysis hemoglobin (g/dL) | 3× | 11.9 (10.3, 13.8) | 12.0 (10.9, 13.2) | 11.7 (10.7, 12.8) | −0.13 (−0.54, 0.27 ) | 0.17 (−0.30, 0.63) | 0.13 (−0.29, 0.55) | −0.12 (−0.61, 0.37) |
| 6× | 12.1 (10.6, 13.3) | 12.1 (11.0, 13.3) | 11.9 (10.9, 13.1) | 0.04 (−0.37, 0.44 ) | 0.01 (−0.42, 0.43) | |||
| ESA/hemoglobin ratioa | 3× | 732 (274, 2948) | 1113 (227, 2778) | 597 (193, 2783) | 2.55 (−23.5, 37.5) | −1.19 (−33.3, 46.5) | −18.5 (−41.7, 13.8) | 42.7 (−7.49, 120.0) |
| 6× | 743 (365, 2381) | 651 (175, 2669) | 632 (231, 3348) | 1.33 (−24.3, 35.7) | 16.3 (−17.1, 63.2) | |||
| Monthly IV iron (mg)a | 3× | 200 (0, 600) | 175 (0, 400) | 200 (0, 500) | 7.87 (−60.1, 191.5) | −63.6 (−88.33, 13.62) | 162.2 (4.16, 560.0 ) | −66.6 (−88.5, −2.51)* |
| 6× | 100 (0, 400) | 100 (0, 200) | 100 (0, 250) | −60.7 (−85.2, 4.56) | −12.32 (−65.66, 123.9) | |||
| Predialysis transferrin (%) | 3× | 23 (14, 34) | 21.5 (15, 35) | 25.5 (14, 38) | 1.07 (−4.58, 2.44) | 4.13 (−0.12, 8.39) | 1.46 (−2.69, 5.60) | −1.00 (−6.28, 4.27) |
| 6× | 27 (10, 38) | 28 (14, 43) | 28 (13, 37) | 3.06 (−0.42, 6.54) | 0.45 (−3.64, 4.54) | |||
| Predialysis ferritin (ng/mL) | 3× | 258 (68, 719) | 336 (123, 586) | 390 (179, 810) | 14.1 (−59.4, 87.6) | 17.7 (−82.9, 118.2) | 46.9 (−43.8, 137.5) | −64.4 (−177.1, 48.7) |
| 6× | 420 (80, 902) | 362 (95, 902) | 361 (29, 730) | 31.8 (−40.9, 104.4) | −17.5 (−107. 1, 72.0) | |||
a% changes in geometric means for ESA dose, ESA/hemoglobin ratio and monthly IV iron.
*P < 0.05.
Iron-related measures
Tables 2 and 3 show the total monthly IV Fe doses administered in each trial by group, comparing the percent change in geometric means from baseline to Months 4 and 12 for the daily and nocturnal trials, respectively. There was no treatment effect on the total monthly dose of IV Fe administered to subjects in the daily trial, but in the nocturnal trial patients in the 6× group (Table 3) received lower amounts of IV Fe at Month 12 compared with the 3× group. There was no treatment effect on serum ferritin levels or transferrin saturation in either trial.
Effect on Hb concentration
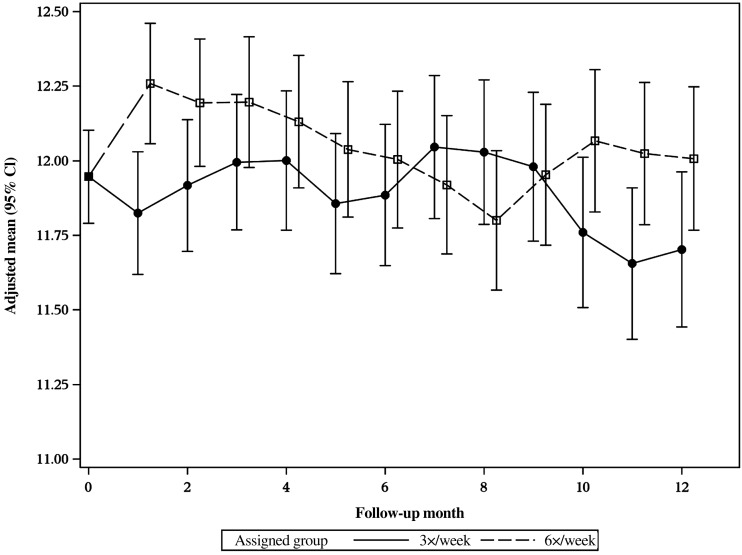
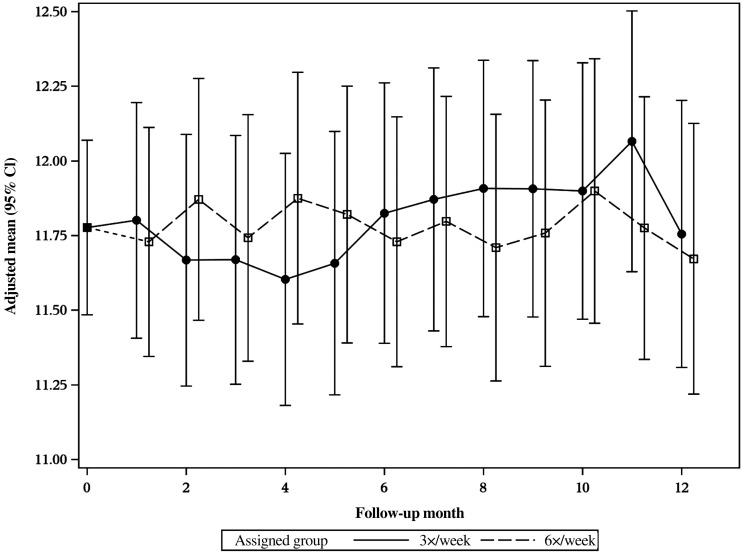
Figures 5 and 6 show the baseline and monthly follow-up, adjusted-mean Hb levels for the 6× and 3× treatment groups in the daily and nocturnal trials, respectively. In the daily trial, the mean Hb concentration at Month 1 was significantly higher in the 6× group (12.3 ± 0.10 g/dL) compared with the 3× group (11.8 ± 0.11 g/dL, P < 0.05). The increase in Hb concentration did not persist throughout the follow-up, but by the last 3 months of the daily trial the mean Hb concentration was again significantly higher in the 6× group. The difference was small in magnitude, in the range of 0.3 g/dL. In the nocturnal trial, there was no difference in Hb concentration between randomized groups at any point during the follow-up (Figure 6). Tables 2 and 3 list the treatment effect on Hb concentration of frequent HD from baseline to Months 4 and 12 in the daily and nocturnal trials, respectively. There was a significant treatment effect on Hb concentration at Month 12 in the daily trial for the 6× versus the 3× arm (0.3 g/dL; 95% CI: 0.0–0.6, P < 0.05). There was no significant treatment effect on Hb concentrations in the nocturnal trial (Table 3).
FIGURE 5:
The adjusted-mean Hb levels in g/dL for in the daily FHN trial are plotted by month by group for 6× and 3×. The Hb level at F1 is significantly greater in the 6× group (P < 0.02). This significantly increased level is again seen in the 6× group at F10 through 12 when comparing the average level with the 3-month period.
FIGURE 6:
The adjusted-mean Hb levels in g/dL for in the nocturnal FHN trial are plotted by month by group for 6× and the 3×. There are no significant differences in Hb levels between the groups across the 12 month study.
To explore the impact of the volume status on changes in Hb concentration, the levels of serum albumin and change in predialysis weights were also examined at the same time points that are depicted in Figures 5 and 6 (results described in detail elsewhere [16]). In the daily trial, at Month 1, there was a non-significant increase in serum albumin in the 6× compared with the 3× group. This increase became significantly greater in the 6× group by Month 3. In addition, there was a significant decrease in predialysis weight in the 6× group at Month 1. By Month 12 predialysis weights were no longer significantly lower in the 6× group and the difference in serum albumin was no longer observed. In the nocturnal trial, differential changes in serum albumin and predialysis weight between the arms were not observed [17].
Subgroup analysis
Subgroup analyses examining the roles of ESRD vintage, age, residual renal function and the presence of diabetes as effect modifiers in the daily trial are shown in Table 4. The effect of frequent HD on reducing ESA dose was slightly but not significantly more pronounced in patients with diabetes (P < 0.07).
Table 4.
Treatment effect on percent change to 12 months in geometric mean EPO dose subgroup analyses in daily trial
| Subgroup factor | Subgroup | Estimated effect | Lower 95% CI limit | Upper 95% CI limit | Interaction P-value |
|---|---|---|---|---|---|
| ESRD | <4 years | −4.64 | −32.13 | 34.00 | 0.94 |
| Vintage | ≥4 years | −30.55 | −53.80 | 4.41 | |
| Age | ≤50 years | −19.73 | −44.38 | 15.84 | 0.38 |
| >50 years | −12.52 | −39.45 | 26.38 | ||
| Baseline GFR | GFR ≤1 mL/min | −22.93 | −43.13 | 4.45 | 0.72 |
| GFR >1 mL/min | 12.98 | −30.24 | 82.97 | ||
| Diabetes | No diabetes | 0.46 | −27.69 | 39.57 | 0.07 |
| Diabetes | −37.80 | −58.29 | −7.24 |
DISCUSSION
The results show that treatment with HD 6× per week compared with the conventional frequency of 3× per week does not result in a significant reduction in ESA dose. In the daily trial, there was a significant increase in predialysis Hb concentration in patients being dialyzed 6× per week; however the small magnitude of this increase (0.3 g/dL) may not be clinically important. Despite a non-significant trend for a lower dose of ESA in the 6× group and a slightly greater Hb concentration, the ESA/Hb ratio was not significantly impacted by frequent HD. In the nocturnal trial, neither ESA dose nor the Hb concentrations were affected significantly by frequent HD.
The Hb concentrations at baseline were similar between the treatment arms in both the daily and the nocturnal trials, with mean values in the range of 11.6–12.0 g/dL. These values were in the range recommended by KDOQI evidence-based guidelines in force during the 2006–10 period when the study was active [13]. Since then, evidence of harm when ESAs are prescribed to achieve goal Hb concentrations of >13 g/dL has changed clinical practice [18, 19]; lowering both the goal and the achieved levels of Hb [20]. Thus, while the FHN trials show no evidence that increasing dialysis frequency improves Hb concentration when the baseline Hb is 11.6–12 g/dL, it is not known if the same would be true with a lower baseline Hb level.
In the daily trial, there was a significant increase in Hb concentration in the 6× group by Month 1. The increase was modest but was present at Month 2 as well. Since Hb concentration is determined not only by the Hb content, but also by the intravascular volume, it is important to examine the effect of more frequent HD on the amount of fluid gained between treatments. In the daily trial, there was a significant reduction in predialysis weights and a significant increase in predialysis serum albumin at Month 1 in the 6× compared with the 3× group [17]. By 3–4 months, the difference in Hb concentrations between the 6× and 3× groups was no longer observed; however, the significantly higher Hb concentration was again observed by Months 10–12 in the 6× group. These findings suggest that the early effect of frequent HD on Hb concentration may have been caused by reduced hemodilution of daily dialysis. It remains unclear whether the significantly higher Hb concentration by Month 12 was related to hemoconcentration, improved erythropoiesis or both. In the nocturnal trial, there was no initial relative rise in Hb concentration observed with frequent HD, nor was there a difference in predialysis weight or serum albumin [17].
The primary, prespecified anemia-related outcome in both daily and nocturnal trials was the weekly ESA dose. We reported dose per week rather than dose per treatment to adjust for differences in weekly treatment frequency in the two arms of the trials. For comparison with the general HD population, at baseline when all subjects were receiving HD 3× per week, the average ESA dose per treatment in the daily trial was ∼5000 units; while in the nocturnal trial it was ∼3800 units. In both trials, the range in baseline ESA doses was quite large, from 0 to >70 000 units per treatment. Although many previous studies looking at ESA dosing in frequent HD studied the impact of frequency on mean ESA dose; such wide variation in prescribed doses results in very large standard errors and difficulty in properly assessing the impact of the treatment intervention on ESA dose. In this report, to minimize this problem, the Windsorized mean of the log of ESA dose was calculated as the measure for comparison [15]. In the daily trial, the total weekly dose of ESA calculated in this fashion was not affected by frequent dialysis, although a non-significant trend toward a decrease in ESA dose in the 6× group was found, along with a trend in the opposite direction (increase in dose) in the 3× group. In the nocturnal trial, there also was no significant effect of frequent HD on weekly ESA dose and in the 6× group at Month 12 the trend was for an increased, rather than a decreased amount of ESA being given.
Common practice has been to prescribe ESA dose to a target Hb level. When higher ESA doses are required to achieve a given Hb concentration, this may reflect relative resistance to the effectiveness of ESAs. One method of assessing this is to calculate an ‘erythropoietin resistance index’, which is the ratio of ESA dose to blood Hb concentration [16]. ESA resistance has been linked to poor patient outcome and to a covert inflammatory state [16]. Previous studies have shown that resistance to ESAs may be influenced by the amount of HD provided [6]. We did not find a significant effect of more frequent HD on ESA responsiveness in either trial. A statistically higher Hb level at Month 12 with a lower (albeit non-significant) ESA dose in the 6× group resulted in a non-significant trend for decrease in the ESA/Hb ratio in the daily trial, but it was not statistically significant. There was no effect of frequent HD on the ESA/Hb ratio in the nocturnal trial.
Iron availability is a key factor in the management of anemia in HD patients [21]. Recurring blood loss in the dialysis circuit may induce iron deficiency, impairing the ability of HD patients to maintain adequate red cell stores [8]. Six times per week treatments might double the amount of blood loss associated with HD and increase the need for iron replacement. Despite these theoretical considerations, in the daily trial, frequent HD had no effect on the administered iron dose. Further, the nocturnal trial, there was significant reduction in the dose of iron being administered at Month 12 in the 6× group compared with the 3× controls. The reason for the lower need for iron in the 6× group in the nocturnal trial is not clear, but certainly argues against the hypothesis that frequent HD increases dialysis-associated blood loss. As further evidence that frequent HD did not impact importantly on anemia management, serum iron, transferrin saturation, total iron-binding capacity and ferritin did not significantly change during the follow-up and were not influenced by the intervention in either trial.
In the daily trial, we hypothesized that patients in several subgroups might be more or less sensitive to the effect of frequent HD on anemia management. The preselected subgroups examined included diabetes, age, residual renal function and ESRD vintage. In these analyses, the only positive effect found was for the presence of diabetes. In those patients with diabetes, there was a significant treatment effect of lower ESA dose in the 6× group by Month 12 compared with patients on HD 3× per week. In contrast, an impact of frequent HD on ESA dose was not found in non-diabetic subjects. This result is intriguing as another clinical trial exploring dialysis membrane effects has demonstrated selective improvement in diabetic subjects [22].
There are strengths and limitations to these trials. The FHN trials are the largest randomized studies of the treatment effect of more frequent HD. In the daily trial, the sample size was sufficient to demonstrate significant improvement in the designated primary outcomes pertaining to the cardiac mass and the quality of life [10]. This provides support for interpreting the secondary outcome of anemia in the daily trial. The wide range in ESA doses was addressed by analyzing the change in the log of the ESA dose; a statistical maneuver not utilized in previous trials. The potential role of hemoconcentration with more frequent HD was examined by concomitantly measuring serum albumin and body weight at multiple time points [17]. There were also limitations to these studies. Practice patterns for prescribing ESA to goal Hb levels have changed since this study was performed. Thus, a lower goal Hb level may have had a different outcome. While the daily trial randomized 245 subjects, sufficient to demonstrate significant treatment effects, this is still a relatively small study. In the nocturnal trial, the sample size was even smaller and was not adequate for drawing conclusions with regard to anemia management.
In summary, there was no significant treatment effect of frequent HD on ESA dose in either the daily or nocturnal FHN trial. In the daily trial, there was a non-significant trend toward a reduced ESA dose in the frequent HD group and there was a significant reduction in ESA dose in diabetic patients on frequent HD, suggesting further studies in this subgroup should be performed. In the daily trial, a small, initial increase in Hb concentration in the frequent group was likely due to hemoconcentration. Frequent HD was associated with a higher Hb concentration by Month 12, but the magnitude of this rise was small. Finally, the postulated increased blood losses in the dialysis circuit with more frequent therapy did not appear to be substantial enough to require more iron replacement.
CONFLICT OF INTEREST STATEMENT
This manuscript was published previously in abstract form only at the American Society of Nephrology, November 2011. It has not been previously published in any journal and is not under review in any other journal. Ornt, Larive, Rashid, Hernandez: none to declare. Daugirdas: Honoraria: Gambro. Rastogi: research-DaVita/Rockwell, Amgen, speaker bureau-Takeda/Affymax. Kurella Tamura: advisory board Amgen. Levin: ownership interest: Fresenius, consultant Affymax, Honoraria: Rofar; Affymax; Roche, Aethlon. Kliger: research funding: Amgen, Honoraria: Affymax.
ACKNOWLEDGEMENTS
A list of members of the FHN trial group for each study has been published and is given in Chertow et al. [10] and Rocco et al. [11]. This work was supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases, the Center for Medicare and Medical Services and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter and Dialysis Clinics, Inc. (DCI). Additional support was provided by DaVita, DCI, Fresenius Medical Care, Renal Advantage, Renal Research Institute and Satellite Healthcare.
REFERENCES
- 1.Folkert VW, Meyer TW, Hostetter TH. Anemia therapy in ESRD: time to move on. Clin J Am Soc Nephrol. 2010;5:1163–1164. doi: 10.2215/CJN.03680410. [DOI] [PubMed] [Google Scholar]
- 2.Puñal J, Lema LV, Sanhez-Guisande D, et al. Clinical effectiveness and quality of life of conventional haemodialysis versus short daily haemodialysis: a systematic review. Nephrol Dial Transplant. 2008;23:2634–2646. doi: 10.1093/ndt/gfn010. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Culleton BF, Walsh M, Klarenbach SW. Effect of frequent nocturnal hemodialysis vs. conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 5.Rao M, Muirhead N, Klarenbach S, et al. Management of anemia with quotidian hemodialysis. Am J Kidney Dis. 2003;42(Suppl. 1):S18–S23. doi: 10.1016/s0272-6386(03)00533-x. [DOI] [PubMed] [Google Scholar]
- 6.Ayus JC, Mizani MR, Achinger SG, et al. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. 2005;16:2778–2788. doi: 10.1681/ASN.2005040392. [DOI] [PubMed] [Google Scholar]
- 7.Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44:715–719. [PubMed] [Google Scholar]
- 8.Sargent JA, Acchiardo SR. Iron requirements in hemodialysis. Blood Purif. 2004;22:112–123. doi: 10.1159/000074931. [DOI] [PubMed] [Google Scholar]
- 9.Suri RS, Garg AX, Chertow GM, et al. Frequent hemodialysis network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Levin NW, Beck GJ, et al. Effects of frequent in-center hemodialysis: the frequent hemodialysis network daily trial. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobin J, Cernii A, McLean R, et al. Conversion from epoetin alfa to darbepoetin alfa for management of anaemia in a community chronic kidney disease centre: a retrospective cohort study. Clin Drug Investig. 2011;31:113–120. doi: 10.1007/BF03256938. [DOI] [PubMed] [Google Scholar]
- 13.KDOQI; National Kidney Foundation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47(Suppl 3):S16–S85. doi: 10.1053/j.ajkd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 15.Jensen JL. Use of the geometric average for effective permeability estimation. Math. 1991;23:833–840. [Google Scholar]
- 16.Kanbay M, Perazella MA, Kasapoglu B, et al. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29:1–12. doi: 10.1159/000245041. [DOI] [PubMed] [Google Scholar]
- 17.Kaysen GA, Greene T, Larive B, et al. The effect of frequent hemodialysis on nutrition and body composition: frequent hemodialysis network trial. Kidney Int. 2012;82:90–99. doi: 10.1038/ki.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AK, Szczech L, Tang KL, et al. CHOIR investigators: correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Burdmann EA, Chen CY, et al. TREAT investigators: a trial of darbopoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 20.Arbor Research Collaborative for Health. DOPPS Practice Monitor. http://www.dopps.org/DPM/Default.aspx. (4 January 2013, date last accessed) [Google Scholar]
- 21.Kovesdy CP, Kalantar-Zadeh K. Iron therapy in chronic kidney disease: current controversies. J Ren Care. 2009;35(Suppl 2):14–24. doi: 10.1111/j.1755-6686.2009.00125.x. [DOI] [PubMed] [Google Scholar]
- 22.Locatelli F, Martin-Malo A, Hannedouche T, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–654. doi: 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]