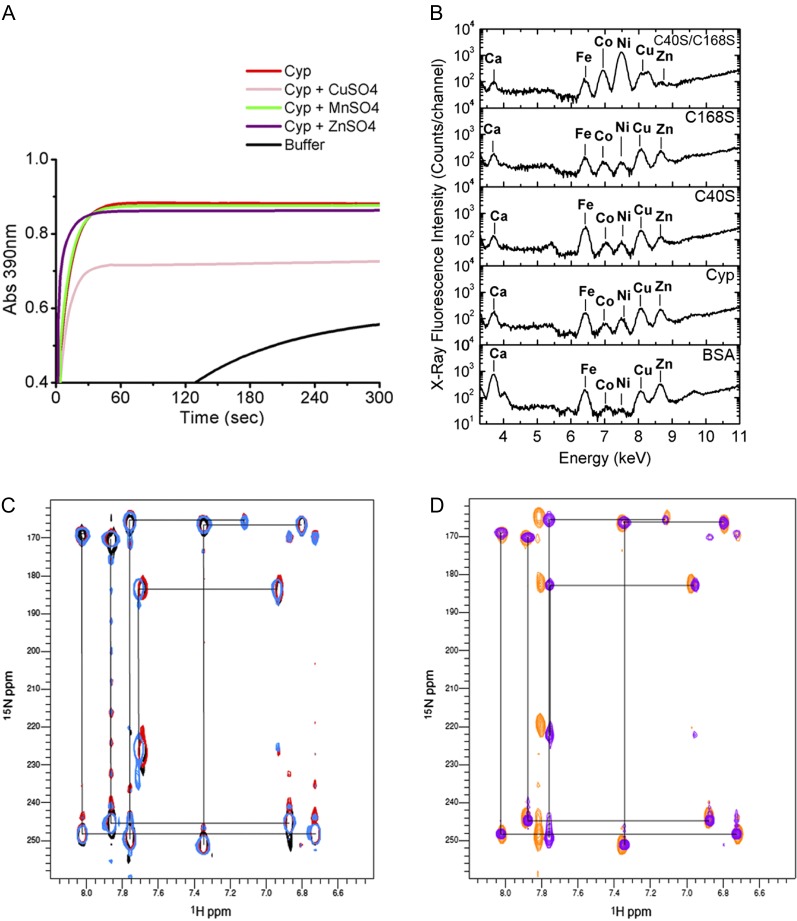
Figure 4.
CsCyp is not a zinc-binding protein. A, PPIase activity of CsCyp in the absence (red) or presence of CuSO4 (pink), ZnSO4 (purple), or MnSO4 (green), showing that CuSO4 inhibits CsCyp activity. The PPIase activity of the buffer alone is shown in black. B, X-ray fluorescence of purified CsCyp and mutants showing the presence of various elements in the samples. No major peak is observed for the wild-type protein compared with the mutant proteins, indicating that CsCyp does not coordinate a specific metal ion. C, Two-bond 2JNH region of 1H-15N SOFAST-HMQC spectra of wild-type CsCyp in the presence of DTT and EDTA (light blue), wild-type CsCyp with Zn2+ (black), and wild-type CsCyp with Zn2+ followed by the addition of EDTA (red). Black lines correlate resonances from atoms within a given imidazole ring. The correlation pattern indicates which of the tautomeric states the His side chain adopts (Pelton et al., 1993). All five His imidazole rings of CsCyp are observed. D, Two-bond 2JNH region of 1H-15N SOFAST-HMQC spectra of wild-type CsCyp in the absence of DTT and EDTA (blue) and of CsCyp C40S/C168S (orange). For clarity, black lines correlating resonances from atoms within a given imidazole ring of wild-type CsCyp only are shown. For details, see text.