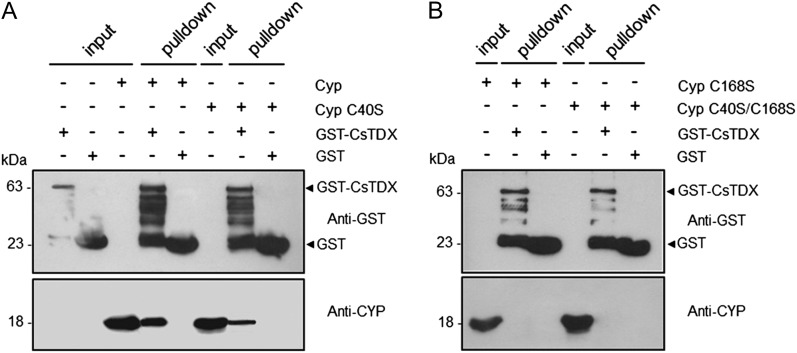
Figure 5.
The conserved Cys residues, Cys-40 and Cys-168, are required for the CsCyp-CsTdx interaction. A GST pull-down assay used GST-CsTdx as bait and the purified wild-type or CsCyp mutants without the 6xHis tag as prey. Protein samples were electrophoresed and probed with the anti-GST or anti-CsCyp serum. A, Wild-type CsCyp, and to a lesser extent the C40S mutant, bind to GST-CsTdx but not to GST alone. B, The interaction between CsCyp and CsTdx is abolished by the C168S and C40S/C168S mutations. Soluble cell extracts of GST-CsTdx, GST alone, or purified CsCyp proteins used as inputs are indicated. The molecular sizes of the corresponding proteins are shown on the left.