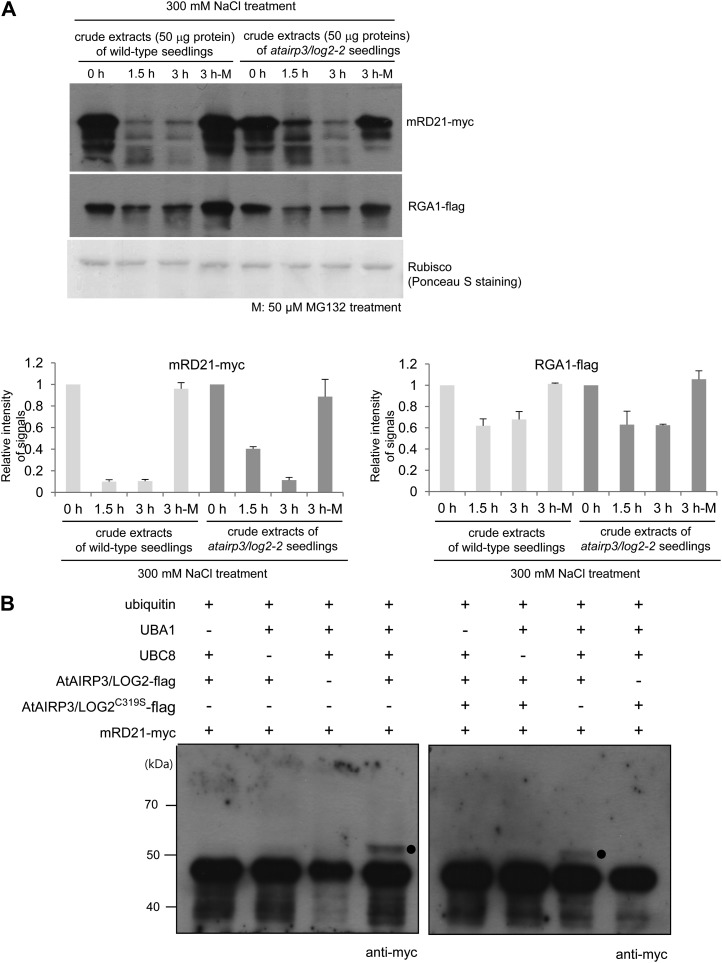
Figure 8.
AtAIRP3/LOG2-dependent degradation of RD21. A, Cell-free degradation assay for mRD21. The mRD21-myc protein was incubated for 0 to 3 h with crude extracts of salt-treated (300 mm NaCl) 10-d-old wild-type or atairp3/log2-2 mutant seedlings in the absence (lanes labeled 0 h, 1.5 h, and 3 h) or presence (lanes labeled 3 h-M) of 50 μm MG132. The time-dependent protein levels were examined by immunoblotting with anti-myc antibody. RGA1, which is known to be regulated by the Ub-26S proteasome pathway, was used as a positive control for proteasome-dependent degradation. The RGA1-flag protein was detected with an anti-flag antibody. Rubisco served as a loading control and was detected by Ponceau S staining. The protein levels were quantified using Multi Gauge version 3.1 software (Fuji Film). B, In vitro ubiquitination of mRD21 by AtAIRP3/LOG2. Recombinant AtAIRP3/LOG2-flag or the AtAIRP3/LOG2-flagC319S derivative was coincubated with mRD21-myc in the presence or absence of Ub, ATP, E1 (Arabidopsis UBA1), and E2 (Arabidopsis UBC8) at 30°C for 1 h. The reaction mixture was analyzed by immunoblotting with an anti-myc antibody. Black circles indicate the shifted high-molecular-mass ubiquitinated protein band.