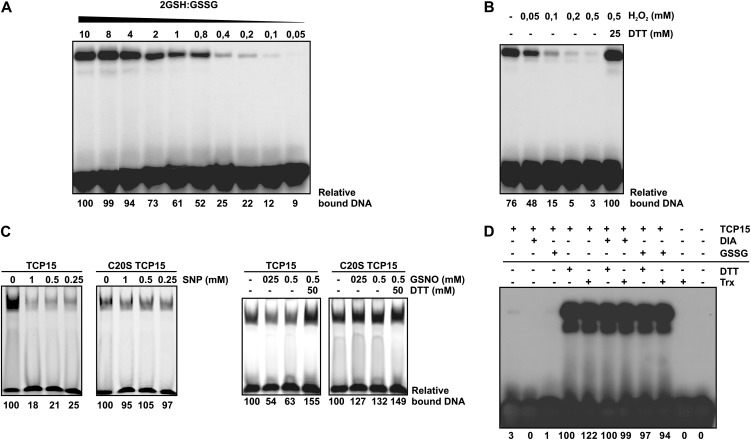
Figure 6.
Cellular redox agents modulate the DNA-binding activity of TCP15. A, TCP15 purified without 2-ME was incubated in buffer at pH 7.5 with different proportions of GSH and GSSG, as indicated. The total concentration of glutathione was kept constant at 10 mm. After incubation, the DNA-binding activity of TCP15 was analyzed by EMSA. B, TCP15 purified without 2-ME was incubated in the presence of different concentrations of H2O2, as indicated, during 1 h at 25°C. After incubation, the DNA-binding activity of TCP15 was analyzed by EMSA. Treatment with DTT, where indicated, was performed during 1 h after the treatment with H2O2. C, TCP15 and its mutant at position 20 of the TCP domain (purified without 2-ME) were incubated in the presence of the NO-producing agents SNP and GSNO at the indicated concentrations during 1 h as specified in “Materials and Methods.” After incubation, the DNA-binding activity of the proteins was analyzed by EMSA. Reversion of the effect of GSNO was assayed by incubating samples with DTT during an additional 20 min. D, TCP15 dialyzed in the absence of reducing agents as described in “Materials and Methods” was incubated in the presence of diamide (DIA), GSSG, or DTT. Alternatively, the protein was incubated with 13 ng µL−1 purified thioredoxin and thioredoxin reductase in the presence of 0.55 mm NADPH (Trx). Where indicated, the treatments with DTT or the thioredoxin system were applied to protein previously oxidized with diamide or GSSG. After the respective treatments, the DNA-binding activity of TCP15 was analyzed by EMSA. Experiments were repeated at least three times with similar results.