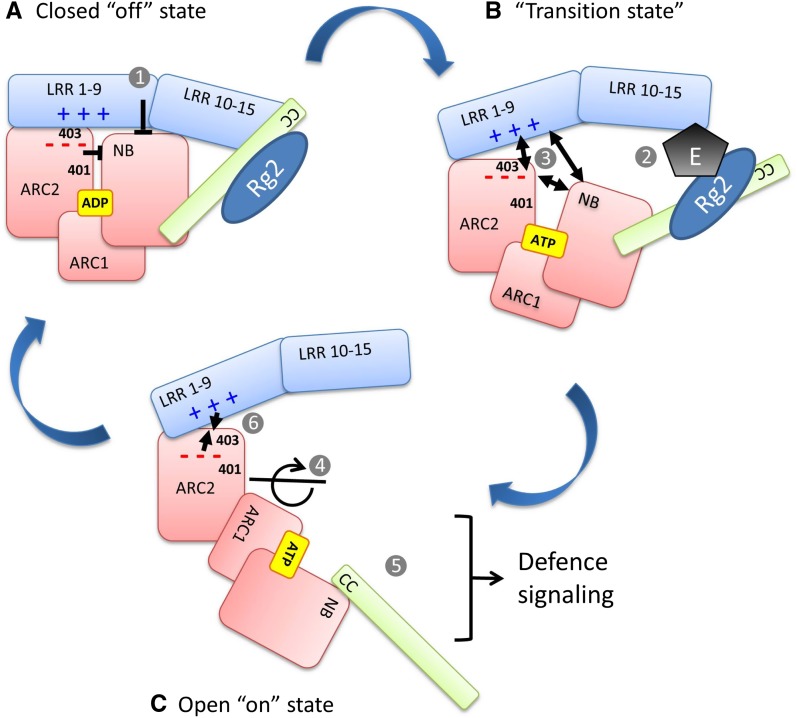
Figure 7.
A mechanistic model of the activation of the NB-LRR proteins Gpa2 and Rx1. This model illustrates the conformational changes underlying the switch between the autoinhibited “off” state (A) and the active-signaling “on” state (C) of the NB-LRR immune receptors Gpa2 and Rx1. The autoinhibited state (A) is stabilized via interactions of the ADP-bound NB and ARC2 with each other and their interface with the LRR. The differential effect of the residues at positions 401 and 403 on elicitor-dependent and elicitor-independent activation is due to their presence in two distinct interfaces. B, Disruption of these domain interfaces by the elicitor (E), potentially facilitated by the presence of a guarded host protein, RanGAP2 (Rg2), at the N-terminal domain (CC), and the exchange of ADP for ATP when the protein is in a transition state allows the NB-ARC1 unit to rotate away from the ARC2 and expose surfaces required for signaling (C). The complementary-charged surfaces of the ARC2 and LRR (– – – and + + +) could accelerate the reassociation of the ARC2 and LRR when the protein returns to the resting state. The numbers in the figure are referred to in the discussion. [See online article for color version of this figure.]