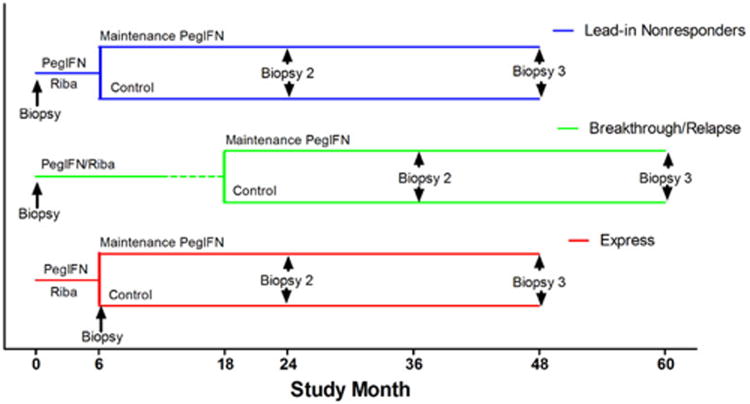
Figure 1.
Design of the HALT-C Trial, showing the source of the three cohorts of patients used in morphometric analyses of fibrosis. Lead-in nonresponder patients (blue line) were those who remained viremic the lead-in phase of Peginterferon/ribavirin for 6 months and were then randomized to maintenance peginterferon or the untreated control group. They had baseline liver biopsies before the lead-in phase and approximately 24 and 48 months later. Breakthrough/relapse patients (green line) developed undetectable HCV RNA during the lead-in phase of peginterferon/ribavirin and completed 12 months of therapy and 6 months of follow-up. Those who became viremic while still receiving therapy (breakthrough) or during follow-up (relapse) were randomized to maintenance or untreated control groups. They had baseline biopsies before the lead-in phase and approximately 36 and 60 months later. Express patients (red line) received the equivalent of the lead-in phase outside the trial but remained viremic. Most had baseline biopsies immediately before randomization to maintenance peginterferon or the control group. Subsequent biopsies were at 18 and 42 months after randomization.