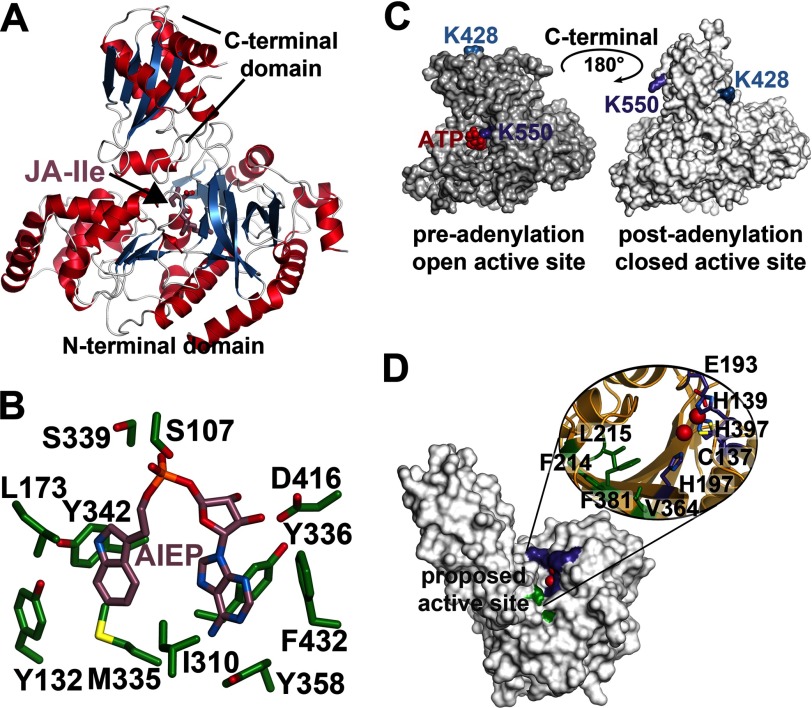
FIGURE 3.
Structural overview of the GH3 acyl-amido synthetases and peptidyl hydrolase ILL. A, overall structure of AtGH3.11/JAR1 (Protein Data Bank code 4EPL) (95). B, active site view of the VvGH3.1 protein in complex with the non-hydrolyzable adenylation intermediate analog adenosine 5′-[2-(1H-indol-3-yl)ethyl]phosphate (Protein Data Bank code 4B2G) (96). C, surface representations of AtGH3.12/PBS3 showing the rotation of the C-terminal domain during the pre-adenylation open active site conformation (Protein Data Bank code 4EWV) (95) and the post-adenylation closed active site conformation (code 4EPM) (95). D, surface view of the AtILL2 peptidyl hydrolase (Protein Data Bank code 1XMB) (99). Residues corresponding to the apolar pocket proposed as the substrate-binding site are colored green, and the residues forming the canonical metal-binding site are colored purple.