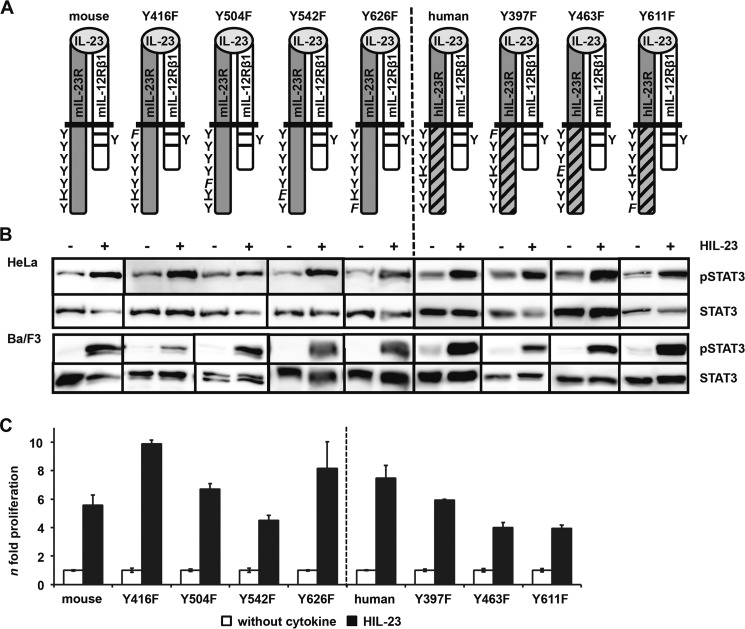
FIGURE 3.
Analysis of postulated STAT3 binding site within the cytoplasmic region of the murine IL-23R. A, IL-23R mutants (mouse Y426F, Y504F, Y542F, and Y626F; human Y397F, Y463F, and Y611F) were generated by site-directed mutagenesis. A scheme of the positions of the resulting tyrosine (Y) to phenylalanine (F) substitutions introduced in the wild-type IL-23R cytoplasmic domain is shown. The second receptor of the IL-23 signaling complex, IL-12Rβ1, remains unmodified. B, p409 expression vectors containing the cDNAs were co-transfected into HeLa cells as indicated. Cells were starved overnight, followed by stimulation with 0.2% HIL-23. Whole cellular lysates have been prepared, and equal amounts of proteins (50 μg/lane) were loaded onto SDS gels. Western blots were performed with antibodies specific for phospho-STAT3. Membranes were reprobed after stripping with STAT3 antibodies. Corresponding stably transduced Ba/F3-gp130 cells were washed three times with PBS and starved for 5 h, followed by stimulation with 0.2% HIL-23 for 10 min. Whole cellular lysates have been prepared and subjected to Western blot analysis as described for HeLa experiments. The presented Western blots originate from different membranes and are therefore separated by black lines. Data are representative of at least two independent experiments. C, stably transduced Ba/F3-gp130 cells were analyzed in a proliferation assay. After 48 h of stimulation with 0.2% HIL-23, proliferation of cells was measured using the colorimetric CellTiter-Blue® cell viability assay. Values represent the mean value of three repetitions and were normalized by subtraction of time point 0 values. For comparison of the independent stably transduced Ba/F3-gp130 cell lines, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. Error bars, S.D.