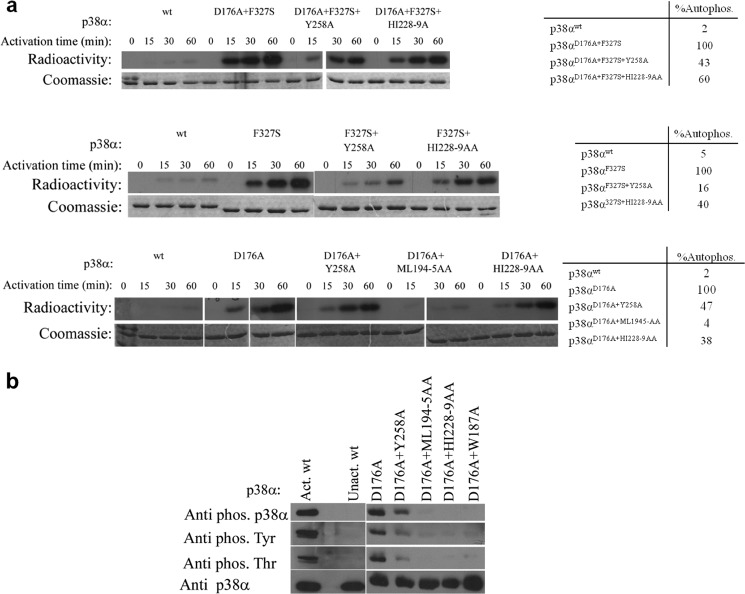
FIGURE 4.
a, in vitro autophosphorylation kinase assay of p38α intrinsically active mutants (p38αD176A, p38αF327S, and p38αD176A+F327S) combined with the DEF pocket mutants (p38αY258A, p38αML194–5AA, and p38α HI228–9AA) at 30 °C as a function of time. Coomassie staining (lower images) verified the amount of p38α in each lane. The radiographs (upper images) show the decreased autophosphorylation capability of the intrinsically active mutants as a result of the different DEF pocket mutations. Qualitative analysis of the autophosphorylation levels of p38α mutants (by counting the bands from the SDS-PAGE using a scintillation counter) is shown in the tables on the right. The autophosphorylation activity of each intrinsically active mutant was normalized to 100%. The results reveal a significant decrease to 16–47% for the Y258A mutant and 38–60% for the HI228–9AA mutant compared with the autophosphorylation level of the intrinsically active mutants. The p38αD176A+ML194–5AA triple mutant shows a decreased autophosphorylation level by 96% compared with p38αD176A. b, Western blot analysis of the p38αD176A mutant combined with the DEF mutants using the anti-p-p38α, anti p-Thr and anti p-Tyr antibodies shows a significant decrease in the intrinsic phosphorylation levels of the DEF mutants compared with those of p38αD176A showing a significant effect of the DEF pocket mutagenesis on the potential autophosphorylation capability of the p38αD176A active mutant. Anti-p38α verified the amount of protein assayed (lower image).