FIGURE 6.
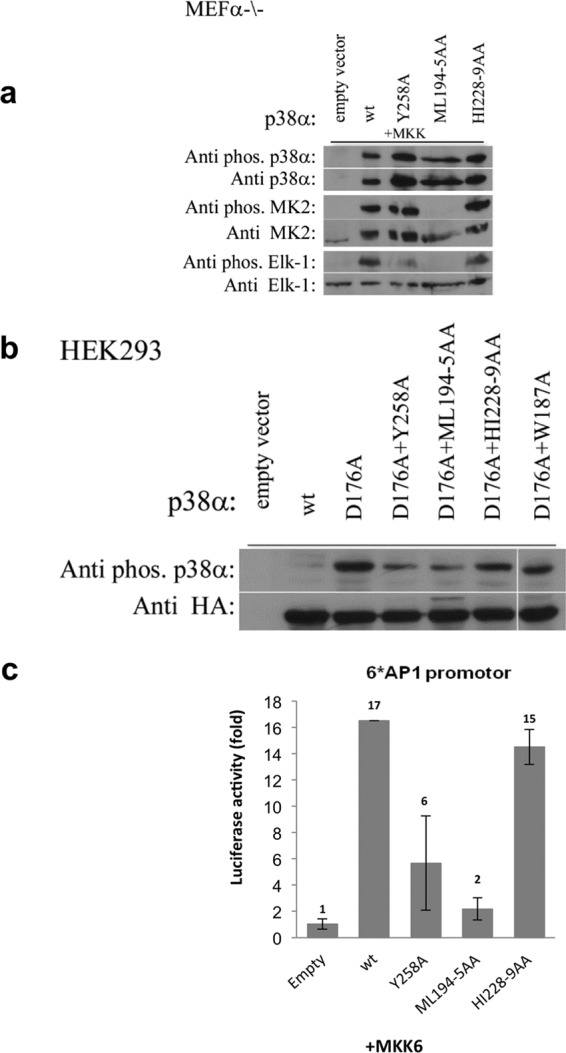
a, MEFα−/− cells were co-transfected with genes of the specified p38α DEF pocket mutants, MKK6EE, and the substrates, Elk-1 and MK2. The cells were harvested after 48 h for Western blot analysis using antibodies that specifically recognize the phosphorylated forms of p38α, Elk-1, and MK2 (upper panels). The blots were stripped and re-incubated with antibodies against p38α, Elk-1, and MK2 (lower panels). Analysis reveals that the Y258A mutation resulted in a notable decrease in phosphorylation levels of Elk-1 but not of MK2. The HI228–9AA mutant shows a slight decrease in the phosphorylation levels of Elk-1 but no effect on the MK2. Conversely, for the ML194–5AA mutant, no phosphorylation was observed for both substrates. These results show high coloration to the in vitro kinase assay. b, the autophosphorylation capabilities of p38αD176A combined with the DEF pocket mutants were examined in HEK293 cells. Cells were transfected with the relevant HA-tagged p38α mutant genes and 48 h post-transfection the cells were harvested for Western blot analysis. The results reveal that p38αD176A is spontaneously phosphorylated in cells but levels decrease by mutating DEF pocket residues or with W187A, verifying the involvement of these residues in autophosphorylation. c, to examine if the DEF pocket is required for natural activation of the p38α cascade, MEFα−/− cells were co-transfected with the genes of the specified p38α DEF pocket mutants, MKK6EE, the AP-1-luciferase reporter gene, and a Renilla luciferase gene. 48 h post-transfection, the cells were harvested and dual luciferase activity was measured. The results clearly indicate a significant decrease in luciferase activity of p38αY258A but not the p38αHI228–9AA mutant compared with p38αwt, indicating that the DEF pocket in p38α is required for its transcriptional activity. The results are the average of two independent experiments and normalized to the activity of empty vector-transfected cells (left bar).
