FIGURE 7.
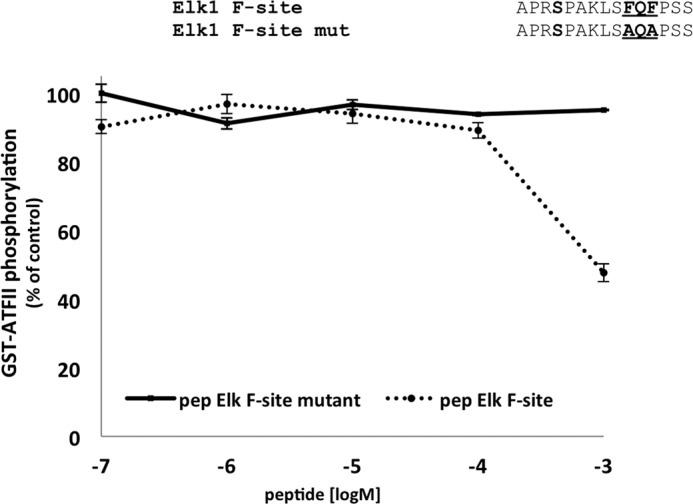
Inhibition of the ATFII phosphorylation activity of dually phosphorylated p38αwt by Elk-1 F-site derived peptides. p38αwt was initially activated in vitro by MKK6EE and the phosphorylation levels of ATFII in the presence of increasing concentrations of the peptides (between 0.1 μm to 1 mm) were monitored by a paper-spotted kinase assay. The Elk-1 F-site-mutated peptide, where the FQF motif was mutated into AQA, was used as a negative control (the full sequence is shown in the upper part of the figure). The phosphorylation levels of ATFII in the presence of 0.1 μm Elk-1 F-site mutant was normalized to 100%. These results clearly show a significant decrease in ATFII phosphorylation to ∼50% by the F-site peptide with no apparent effect by the negative control peptide.
