FIGURE 4.
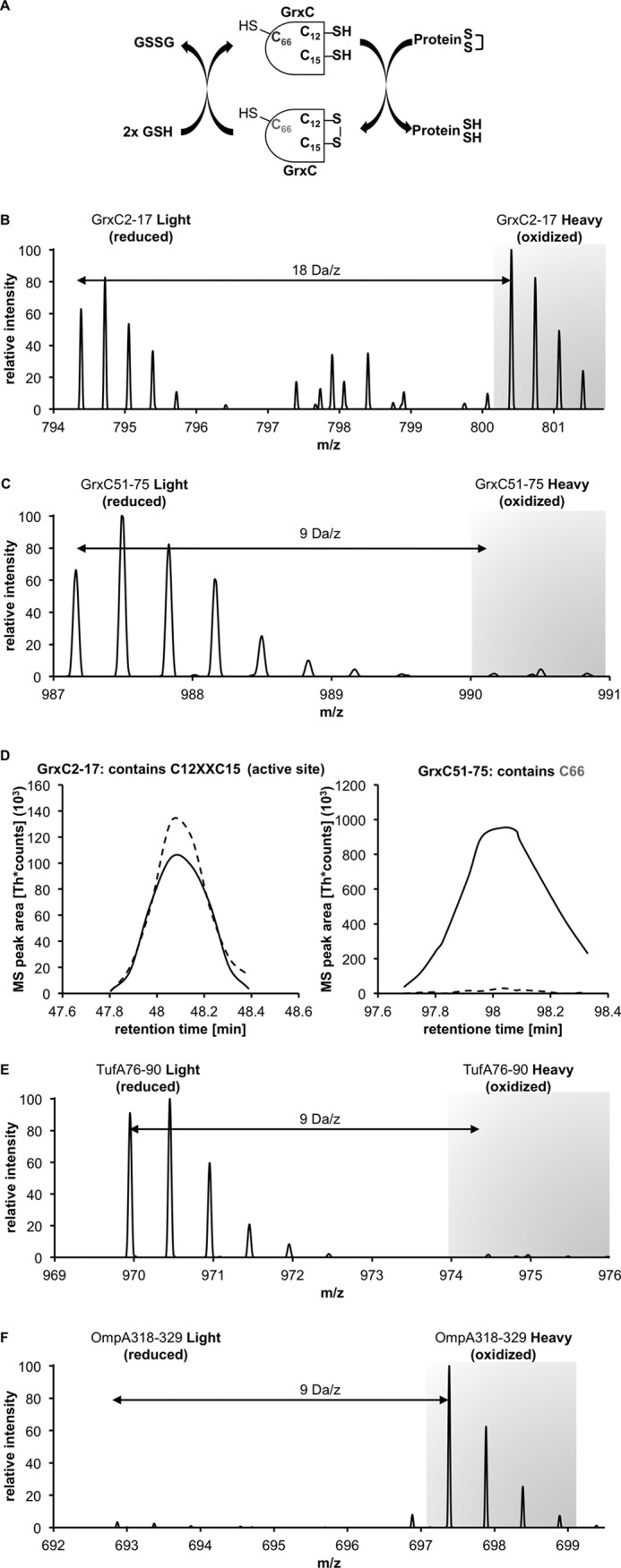
Oxidation state of cysteines of cytosolic and periplasmic proteins under nonstress growth conditions. A, GrxC (glutaredoxin 3) is an enzyme that forms a disulfide bond between Cys-12 and Cys-15 during its active cycle. The nonconserved C66 is not involved in the catalytic cycle. B, NOxICAT method allows us to observe the steady state of this enzyme. The parental ion MS of the peptide identified as GrxC 2–17 shows the reduced and oxidized form 2 × 9 Da = 18 Da apart. The intensities correspond to the redox state of this antioxidant enzyme system. C, peptide containing C66, however, is almost exclusively present in its reduced form. D, redox state of both peptides can be quantified with MSQuant using the elution profile of these peptides. The solid line represents the reduced (light) form, and the dashed line represents the oxidized (heavy) form. E, NOxICAT detects cytosolic cysteines that are not involved in catalytic cycles, such as Cys-82 from TufA (EF-Tu, one of the highest abundant proteins in the E. coli cytoplasm) almost exclusively in their reduced form, confirming the dogma of a reducing cytosol. F, OmpA, a major periplasmic protein is detected as fully oxidized in the NOxICAT assay, demonstrating the ability of NOxICAT to detect structural disulfides present in periplasmic proteins.
