Abstract
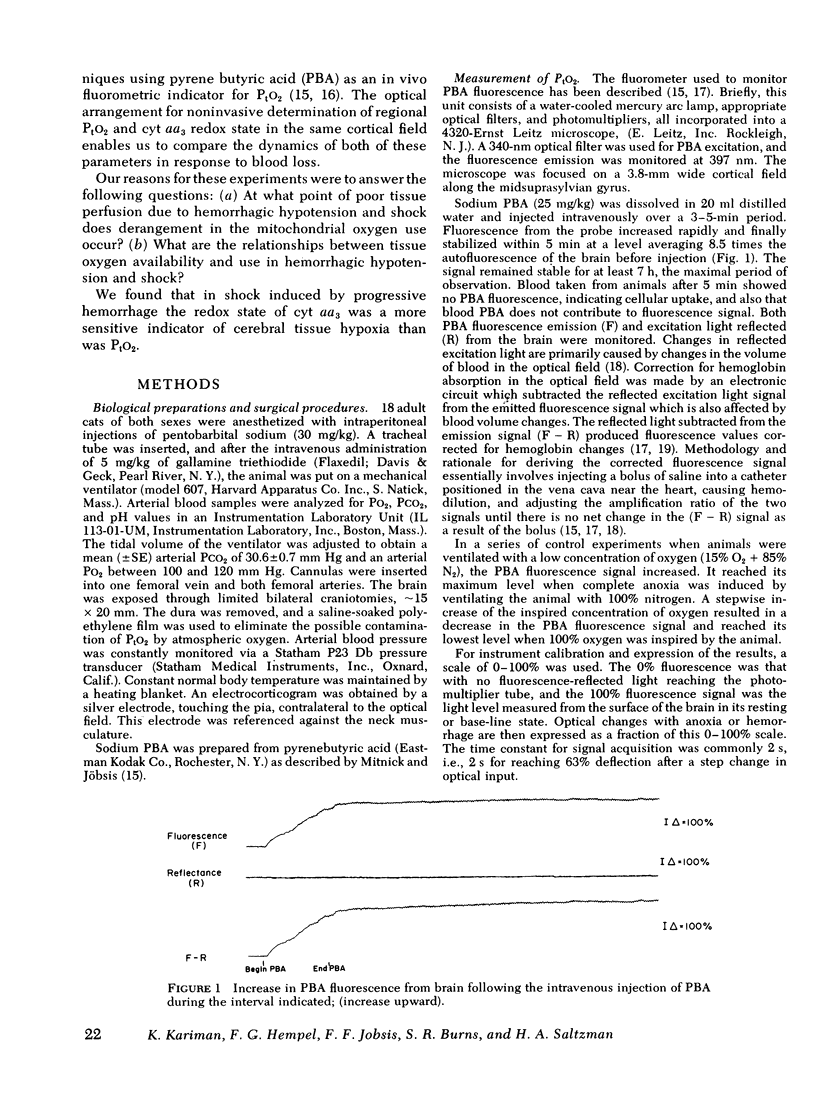
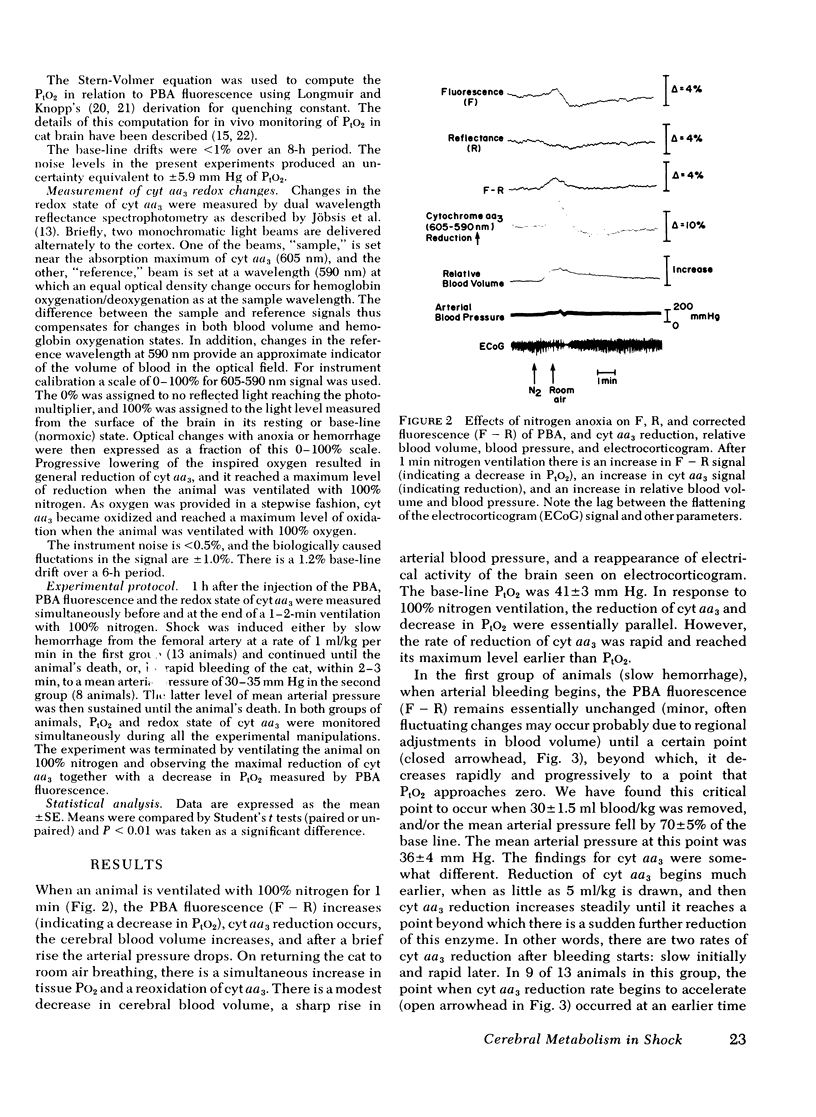
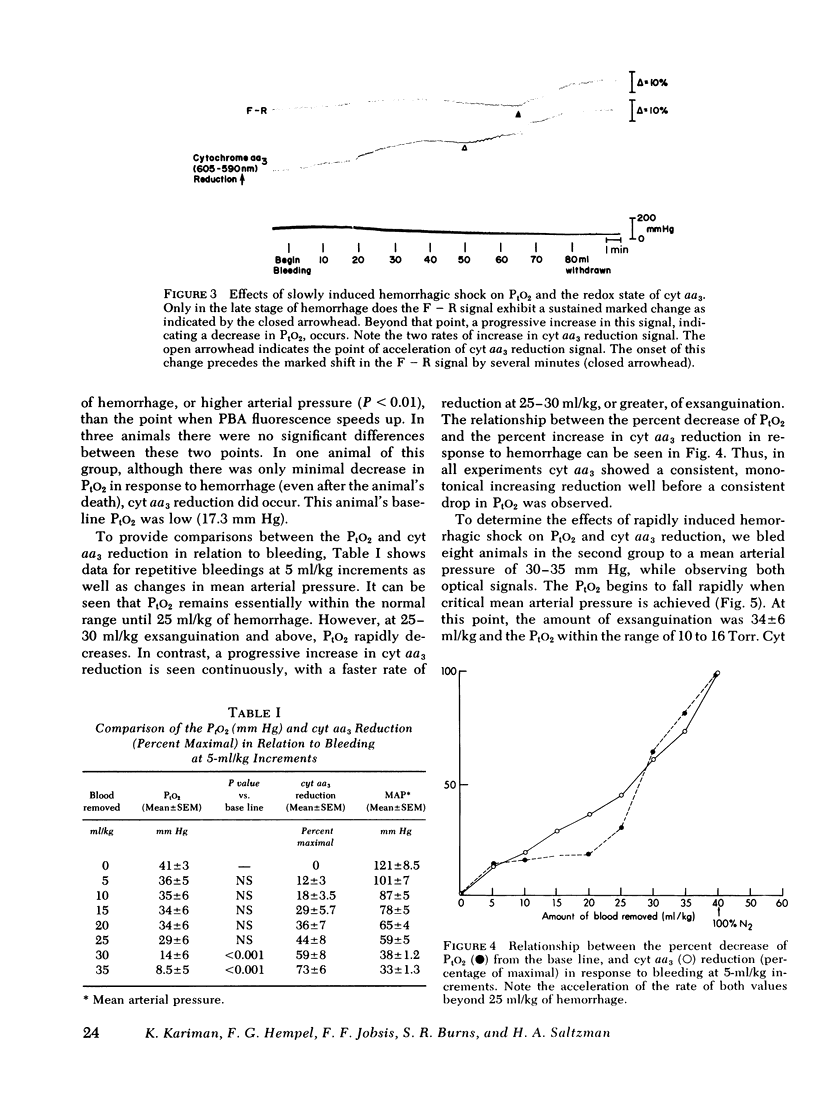
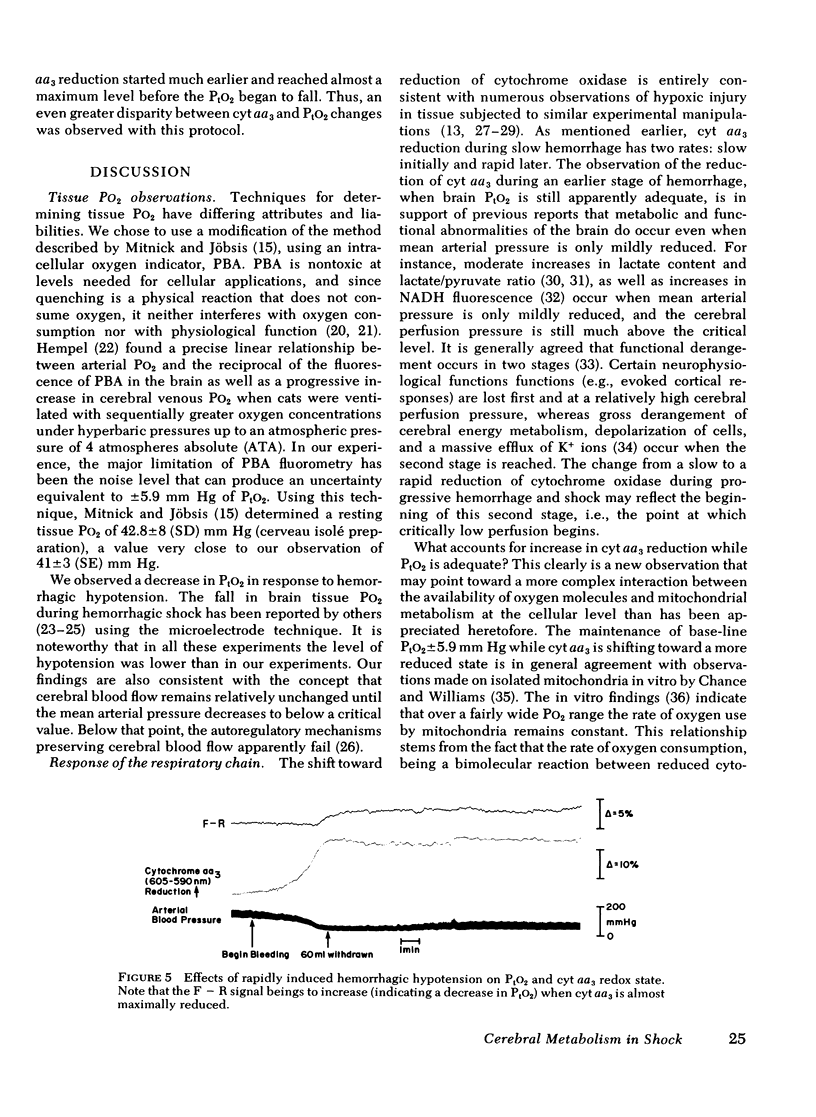
To assess the adequacy of oxygen availability and utilization within the cerebral cortex in vivo, we have measured the partial pressure of oxygen in tissue (PtO2), as well as the reduction oxidation state of cytochrome c oxidase (cyt aa3) during shock induced by slow or rapid hemorrhage in anesthetized cats. PtO2 was measured with pyrenebutyric acid-generated fluorescence in cerebral cortical cells. Cyt aa3 redox state was measured by the absorption of monochromatic light at 605 nm absorption peak of the enzyme reflected from the same cortical field. The PtO2 remained within the normal range until either 30 +/- 1.5 ml blood/kg was removed or the mean arterial pressure fell by 70 +/- 5% of base line. Beyond either point, the PtO2 fell rapidly to a low value approximating zero. By contrast, the reduction of cyt aa3 began early when as little as 5 ml blood/kg was removed. Thereafter, the shift toward reduction was progressive and continuous with a slow rate at first and a rapid rate later. This accelerated rate of cyt aa3 reduction preceded the rapid fall of PtO2. We concluded that, under these experimental conditions, cyt aa3 reduction is a much earlier and more sensitive indicator of perturbed intracellular aerobic metabolism due to hemorrhage that is PtO2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup J., Symon L., Branston N. M., Lassen N. A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977 Jan-Feb;8(1):51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Fujishima M. The metabolic mechanism of cerebral blood flow autoregulation in dogs. Jpn Heart J. 1971 Jul;12(4):376–382. doi: 10.1536/ihj.12.376. [DOI] [PubMed] [Google Scholar]
- Harbig K., Chance B., Kovách A. G., Reivich M. In vivo measurement of pyridine nucleotide fluorescence from cat brain cortex. J Appl Physiol. 1976 Oct;41(4):480–488. doi: 10.1152/jappl.1976.41.4.480. [DOI] [PubMed] [Google Scholar]
- Hempel F. G., Jöbsis F. F. Comparison of cerebral NADH and cytochrome aa3 redox shifts during anoxia or hemorrhagic hypotension. Life Sci. 1979 Sep 24;25(13):1145–1151. doi: 10.1016/0024-3205(79)90136-x. [DOI] [PubMed] [Google Scholar]
- Hempel F. G. Oxygen tensions measured in cat cerebral cortex under hyperbaric conditions. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jan;46(1):53–60. doi: 10.1152/jappl.1979.46.1.53. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Keizer J. H., LaManna J. C., Rosenthal M. Reflectance spectrophotometry of cytochrome aa3 in vivo. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):858–872. doi: 10.1152/jappl.1977.43.5.858. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977 Dec 23;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M., Vitale A., Vreman H. Intracellular redox changes in functioning cerebral cortex. I. Metabolic effects of epileptiform activity. J Neurophysiol. 1971 Sep;34(5):735–749. doi: 10.1152/jn.1971.34.5.735. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Oxidative metabolism at low PO 2 . Fed Proc. 1972 Sep-Oct;31(5):1404–1413. [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Knopp J. A., Longmuir I. S. Intracellular measurement of oxygen by quenching of fluorescence of pyrenebutyric acid. Biochim Biophys Acta. 1972 Sep 15;279(2):393–397. doi: 10.1016/0304-4165(72)90158-4. [DOI] [PubMed] [Google Scholar]
- Knopp J. A., Longmuir I. S. Intracellular measurement of oxygen by quenching of fluorescence of pyrenebutyric acid. Biochim Biophys Acta. 1972 Sep 15;279(2):393–397. doi: 10.1016/0304-4165(72)90158-4. [DOI] [PubMed] [Google Scholar]
- Kovách A. G., Dóra E., Hamar J., Eke A., Szabó L. Transient metabolic and vascular volume changes following rapid blood pressure alterations which precede the autoregulatory vasodilation of cerebrocortical vessels. Adv Exp Med Biol. 1977 Jul 4;94:705–711. doi: 10.1007/978-1-4684-8890-6_97. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959 Apr;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lamanna J. C., Jöbsis F. F., Austin G. M., Schuler W. Changes in brain metabolism in the cat in response to multiple brief transient ischemic episodes. Exp Neurol. 1977 May;55(2):304–317. doi: 10.1016/0014-4886(77)90002-4. [DOI] [PubMed] [Google Scholar]
- Ljunggren B., Schutz H., Siesjö B. K. Changes in energy state and acid-base parameters of the rat brain during complete compression ischemia. Brain Res. 1974 Jun 20;73(2):277–289. doi: 10.1016/0006-8993(74)91049-x. [DOI] [PubMed] [Google Scholar]
- Longmuir I. S., Knopp J. A. Measurement of tissue oxygen with a fluorescent probe. J Appl Physiol. 1976 Oct;41(4):598–602. doi: 10.1152/jappl.1976.41.4.598. [DOI] [PubMed] [Google Scholar]
- Maekawa T., McDowall D. G., Okuda Y. Brain-surface oxygen tension and cerebral cortical blood flow during hemorrhagic and drug-induced hypotension in the cat. Anesthesiology. 1979 Oct;51(4):313–320. doi: 10.1097/00000542-197910000-00007. [DOI] [PubMed] [Google Scholar]
- Mayevsky A., Lebourdais S., Chance B. The interrelation between brain PO2 and NADH oxidation-reduction state in the gerbil. J Neurosci Res. 1980;5(3):173–182. doi: 10.1002/jnr.490050302. [DOI] [PubMed] [Google Scholar]
- Mela L., Bacalzo L. V., Jr, Miller L. D. Defective oxidative metabolism of rat liver mitochondria in hemorrhagic and endotoxin shock. Am J Physiol. 1971 Feb;220(2):571–577. doi: 10.1152/ajplegacy.1971.220.2.571. [DOI] [PubMed] [Google Scholar]
- Mitnick M. H., Jöbsis F. F. Pyrenebutyric acid as an optical oxygen probe in the intact cerebral cortex. J Appl Physiol. 1976 Oct;41(4):593–597. doi: 10.1152/jappl.1976.41.4.593. [DOI] [PubMed] [Google Scholar]
- Rehncrona S., Mela L., Chance B. Cerebral energy state, mitochondrial function, and redox state measurements in transient ischemia. Fed Proc. 1979 Oct;38(11):2489–2492. [PubMed] [Google Scholar]
- Rosenthal M., Lamanna J. C., Jöbsis F. F., Levasseur J. E., Kontos H. A., Patterson J. L. Effects of respiratory gases on cytochrome A in intact cerebral cortex: is there a critical Po2? Brain Res. 1976 May 21;108(1):143–154. doi: 10.1016/0006-8993(76)90170-0. [DOI] [PubMed] [Google Scholar]
- Sayeed M. M., Baue A. E. Mitochondrial metabolism of succinate, beta-hydroxybutyrate, and alpha-ketoglutarate in hemorrhagic shock. Am J Physiol. 1971 May;220(5):1275–1281. doi: 10.1152/ajplegacy.1971.220.5.1275. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Zwetnow N. N. Effects of increased cerebrospinal fluid pressure upon adenine nucleotides and upon lactate and pyruvate in rat brain tissue. Acta Neurol Scand. 1970;46(2):187–202. doi: 10.1111/j.1600-0404.1970.tb05615.x. [DOI] [PubMed] [Google Scholar]
- Welsh F. A., Durity F., Langfitt T. W. The appearance of regional variations in metabolism at a critical level of diffuse cerebral oligemia. J Neurochem. 1977 Jan;28(1):71–79. doi: 10.1111/j.1471-4159.1977.tb07710.x. [DOI] [PubMed] [Google Scholar]
- Welsh F. A., O'Connor M. J., Langfitt T. W. Regions of cerebral ischemia located by pyridine nucleotide fluorescence. Science. 1977 Dec 2;198(4320):951–953. doi: 10.1126/science.201026. [DOI] [PubMed] [Google Scholar]
- Welsh F. A., O'Connor M. J., Marcy V. R. Effect of oligemia on regional metabolite levels in cat brain. J Neurochem. 1978 Jul;31(1):311–319. doi: 10.1111/j.1471-4159.1978.tb12464.x. [DOI] [PubMed] [Google Scholar]
- Wiernsperger N., Gygax P., Meier-Ruge W. Changes in cerebrocortical pO2-distribution, rCBF and EEG during hypovolemic shock. Adv Exp Med Biol. 1977 Jul 4;94:605–610. doi: 10.1007/978-1-4684-8890-6_82. [DOI] [PubMed] [Google Scholar]


