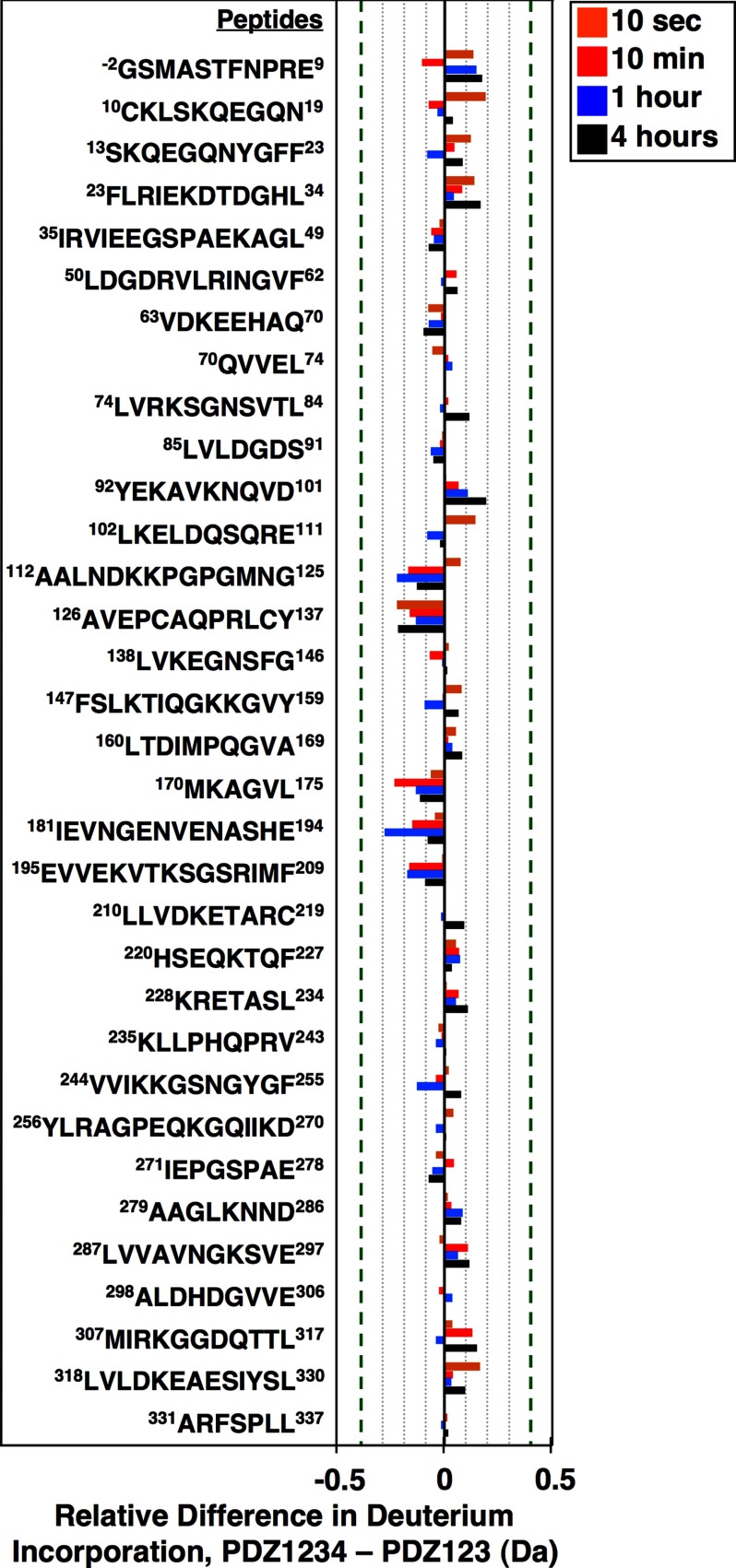
FIGURE 10.
Comparison of the conformations of the PDZ123 regions of PDZ123 and PDZ1234 using hydrogen/deuterium exchange mass spectrometry. Recombinant PDZ123 (residues 1–359) and PDZ1234 (1–458) proteins that also included two additional N-terminal residues (−2Gly and −1Ser, from the cloning vector) were incubated for 10 s (orange), 10 min (red), 1 h (blue), or 4 h (black) in buffer A containing deuterated water. The proton/deuterium exchange reactions were then quenched with low pH (2.5) and temperature (0 °C), and the proteins were digested into peptides, and the average amounts of deuterium incorporated was determined using mass spectrometry. We identified peptides corresponding to 98.2% of the predicted full-length PDZ1234 sequence (supplemental Fig. S2, created using MSTools (55)). Deuterium incorporation plots for the individual peptides presented here are shown in supplemental Fig. S3. The differences in deuterium incorporation for each of the indicated peptides (PDZ1234 to PDZ123) at each time of incubation are expressed in daltons (Da). The error limit for the determination of deuterium incorporation is ±0.4 Da (indicated with vertical dashed green lines (31)).