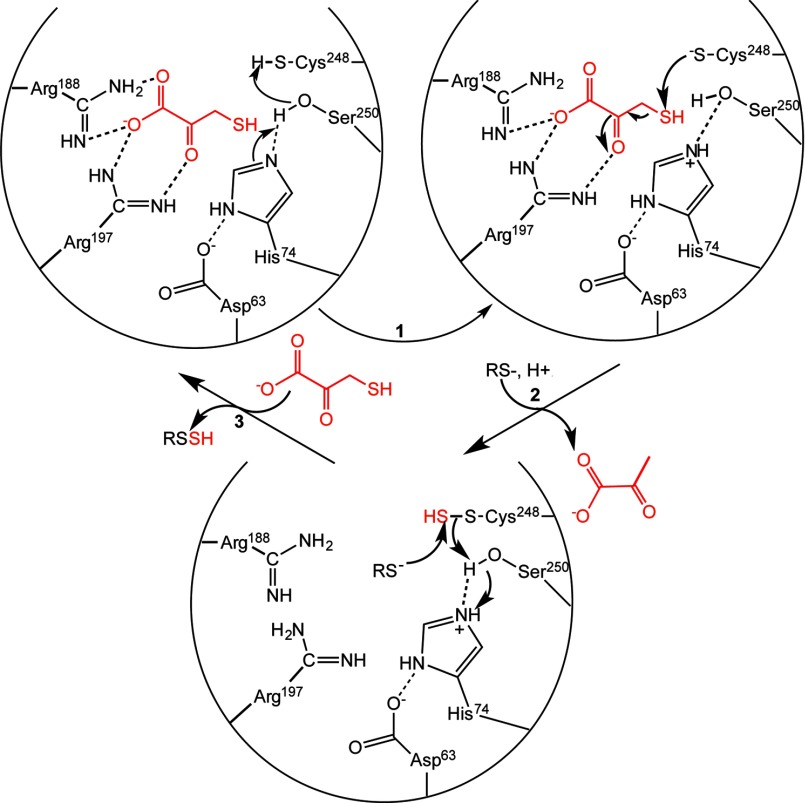
FIGURE 10.
Postulated reaction mechanism of MST. Two arginine residues (Arg188 and Arg197) anchor the substrate carboxylate and carbonyl groups, whereas the catalytic triad (Asp63-His74-Ser250) in MST is postulated to activate Cys248 (step 1) for nucleophile attack on 3-MP (red) to give an enzyme bound persulfide. Release of the pyruvate anion following release and binding of the sulfur acceptor (step 2) set up the second half-reaction. Nucleophilic attack of the acceptor (a small molecule (di)thiol or thioredoxin) on the sulfane sulfur results in formation of the product persulfide, which is released (step 3) to complete the catalytic cycle. For clarity, not all interactions between the hydroxyl group of Ser250 and the substrate discussed in the text or the other steps in which the catalytic triad might participate in acid-base catalysis are shown.