Abstract
♦ Background: 25(OH) Vitamin D [25(OH)D] is the major circulating form of vitamin D and the parameter used to reflect vitamin D status. Patients with chronic kidney disease (CKD) are likely to have low levels of 25(OH)D, and recent observations have linked suboptimal vitamin D status with adverse cardiovascular outcomes, inflammation, insulin resistance, and the rate of progression of renal insufficiency. Little is known about the magnitude of vitamin D deficiency in pediatric patients with stage 5 CKD on chronic dialysis.
♦ Objectives: The aim of the present cross-sectional study was to assess the prevalence of abnormal vitamin D status in children on chronic dialysis.
♦ Methods: Serum 25(OH)D, 1,25(OH)2 vitamin D [1,25(OH)2D], calcium, phosphorus, and parathyroid hormone (PTH) were evaluated in 59 pediatric patients on chronic dialysis. Weekly renal Kt/V and creatinine clearance (CCr) were evaluated as parameters reflecting residual renal function. In these patients, serum 25(OH)D concentrations less than 10 ng/mL were considered deficiency and concentrations of 10 - 30 ng/mL were considered insufficiency.
♦ Results: Of the 59 pediatric patients (mean age: 14.4 ± 5.1 years), 51 (86.4%) were on peritoneal dialysis (PD), and 8 (13.6%) were on hemodialysis. Vitamin D deficiency was found in 32.2% of the patients (n = 19), and vitamin D insufficiency, in 50.8% (n = 30). Patients with serum 25(OH)D concentrations less than 30 ng/mL were older than those with normal 25(OH)D concentrations (15.4 ± 4.5 years vs 9.2 ± 5.1 years, p = 0.000). Patients with 25(OH) D concentrations less than 30 ng/mL had higher PTH levels than did those with normal 25(OH)D concentrations (349.5 ± 318.3 pg/mL vs 142.5 ± 116.9 pg/mL, p = 0.001). In the univariate analysis, there was no correlation between serum 25(OH)D and serum 1,25(OH)2D (r = 0.242, p = 0.064), calcium (r = 0.108, p = 0.415), phosphorus (r = -0.050, p = 0.706), or body mass index (r = -0.046, p = 0.729). In PD patients, serum 25(OH)D was positively correlated with weekly renal Kt/V (r = 0.385, p = 0.005) and CCr (r = 0.443, p = 0.001). In addition, serum 25(OH)D and serum albumin were positively correlated (r = 0.297, p = 0.035) in the PD patients.
♦ Conclusions: The present study found a high prevalence of 25(OH)D deficiency and insufficiency in children on chronic dialysis. Serum 25(OH)D was associated with residual renal function in children on PD. Further studies to evaluate the consequences of vitamin D deficiency and the impact of therapeutic interventions are needed in pediatric CKD patients.
Key words: Chronic kidney disease, 25(OH) vitamin D deficiency, chronic dialysis, residual renal function
Vitamin D plays a central role in skeletal development and has a protective effect against hypertension, cardiovascular morbidity, diabetes mellitus, and cancer (1,2). Vitamin D is sourced from the diet or synthesized in the skin by ultraviolet B sunlight, metabolized in the liver to 25(OH) vitamin D [25(OH)D (calcidiol)], and then in the kidney to the biologically active 1,25(OH)2 vitamin D form [1,25(OH)2D (calcitriol)] under the control of parathyroid hormone (PTH) and fibroblast growth factor (FGF) 23 (3,4). In a dual effect, FGF23 reduces circulating 1,25(OH)2D by suppressing production of Cyp27b1 and stimulating Cyp24 catabolism of 1,25(OH)2D (5). Although 1,25(OH)2D is considered the biologically active form of vitamin D, 25(OH)D is the major circulating form, which is used as the parameter reflecting vitamin D status (4-6). It is known that 25(OH)D activates the vitamin D receptor and circulates in human plasma at approximately 1000 times the concentration of 1,25(OH)2D (7). In addition, because many tissues—including colon, prostate, skin, macrophages, and parathyroid—are recognized to express 1-α-hydroxylase, normal concentrations of 25(OH)D are important for local synthesis of 1,25(OH)2D in those tissues (8,9).
Vitamin D inadequacy results from reduced sun exposure and dietary deficiency, and it is believed to be an epidemic of worldwide proportions in all age groups (10). Currently, for children, severe vitamin D deficiency is defined as a serum 25(OH)D concentration of 5 ng/mL or less, which is associated with an increased risk of rickets and myopathy (11). It has been recommended that a serum 25(OH)D concentration of 15 ng/mL or less be considered a state of vitamin D deficiency. Vitamin D insufficiency is usually defined as a 25(OH)D concentration of 15 - 20 ng/mL, which is associated with osteomalacia (11-13). Although a serum concentration of 25(OH)D greater than 20 ng/mL is considered indicative of vitamin D sufficiency in children, adult data indicate that a level of 32 ng/mL is desirable (11).
Patients with chronic kidney disease (CKD), including dialysis patients, are likely to have low levels of 25(OH) D (14-21). The prevalence of vitamin D insufficiency or deficiency in pediatric CKD patients before dialysis ranges from 60% to 82.1% (15,17). The prevalence of 25(OH)D deficiency is known to be higher in patients on peritoneal dialysis (PD) than in those on hemodialysis (HD) because vitamin D binding protein is lost in peritoneal effluent (20,21). Other studies have shown that the prevalence of 25(OH)D deficiency or insufficiency at the time of renal transplant in adults is 88% (22). In CKD patients, a serum 25(OH)D concentration of less than 30 ng/mL is usually used as the definition of vitamin D deficiency or insufficiency (14-17). In the present study, a serum 25(OH)D concentration of less than 10 ng/mL was considered to represent deficiency and a concentration of 10 - 30 ng/mL was considered to represent insufficiency.
Low levels of serum 25(OH)D, the substrate for the active hormone 1,25(OH)2D, may exacerbate secondary hyperparathyroidism in patients with early CKD (16). Recent observations have linked a suboptimal 25(OH) D status to adverse cardiovascular outcomes and also to the rate of progression of renal insufficiency in CKD (14,23,24). Nonrenal synthesis of 1,25(OH)2D has been described, suggesting that supplementation with cholecalciferol or ergocalciferol in addition to 1,25(OH)2D may have beneficial effects in CKD patients (15,21). The Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend measuring serum 25(OH)D in children with CKD stages 2 - 5 and 5D once annually and supplementing with ergocalciferol or cholecalciferol if serum 25(OH)D is less than 30 ng/mL (25).
Most previous studies of vitamin D status in patients on dialysis were performed in adults, and few reports have looked at serum 25(OH)D in pediatric patients on dialysis. In the present study, we evaluated the prevalence and severity of abnormal vitamin D status in children on chronic dialysis, and the relationship between serum 25(OH)D and other parameters of mineral metabolism, nutrition, and residual renal function (RRF).
Methods
Patients
Pediatric patients on chronic dialysis at two dialysis units located in Seoul, Republic of Korea, were evaluated for inclusion in the study. From January 2011 to March 2011, which corresponds to the winter season in Korea, we examined serum 25(OH)D and 1,25(OH)2D in those patients. Patients were excluded if
they had been on dialysis for less than 3 months;
they had a history of parathyroidectomy;
they had recently experienced an infectious disease, had chronic hepatic disease, or had recently been hospitalized.
The patients had no symptoms of vitamin D deficiency. They did not take any cholecalciferol or ergocalciferol, including any over-the-counter supplements that might contain vitamin D2 or vitamin D3, but they did take active vitamin D sterol according to the K/DOQI clinical practice guidelines for bone metabolism and disease in CKD.
Laboratory Evaluations
Routine chemistry parameters such as serum calcium, phosphorus, albumin, alkaline phosphatase, and hemoglobin were measured by standard automated methods, and serum intact parathyroid hormone (PTH) was assessed by an automated chemiluminescence immunoassay. Serum 25(OH)D and 1,25(OH)2D were measured using a commercially available radioimmunoassay. Blood samples for routine chemistry, 25(OH)D, and 1,25(OH)2D were obtained before a dialysis session in patients on HD. To assess dialysis adequacy and RRF in PD patients, the total weekly Kt/V (renal and peritoneal) and total weekly creatinine clearance [CCr (renal and peritoneal)] were checked using a 24-hour collection of urine and dialysate.
Statistical Analysis
Patient characteristics are presented using frequencies for categorical variables and means for continuous variables. Comparisons between groups (normal vitamin D level compared with vitamin D insufficiency or deficiency) were performed using a t-test or chi-square test for independent samples. Linear regression analyses were used to assess the relationship between serum 25(OH)D and other variables. For all statistical analyses, p < 0.05 was considered to be significant. All statistical analyses were performed using the IBM SPSS software application (version 19: IBM, Armonk, NY, USA).
Results
Patient Characteristics
We evaluated 65 pediatric patients on chronic dialysis for inclusion in the study and recruited 59 stable patients [36 boys, 23 girls; 51 (86.4%) on PD, 8 (13.6%) on HD] with a mean age of 14.4 ± 5.1 years. Table 1 shows the demographics for those patients. All patients were Korean. Their mean serum 25(OH)D was 18.6 ng/mL (range: 2.5 - 150 ng/mL). Hyperparathyroidism had been diagnosed in 46 patients, who were receiving active vitamin D sterols according to their serum PTH and the K/DOQI guideline.
TABLE 1.
Characteristics of the Pediatric Patients on Chronic Dialysis
Vitamin D Status
In the study cohort, we found that 83.0% had serum 25(OH)D concentrations below 30 mg/mL, consistent with vitamin D deficiency or insufficiency. In 19 patients (32.2%), serum 25(OH)D was less than 10 mg/mL, consistent with vitamin D deficiency, and only 10 patients (16.9%) had a physiologically appropriate serum 25(OH) D concentration.
Table 2 shows the characteristics of the patients according to 25(OH)D level. Patients with vitamin D deficiency or insufficiency were older than those with normal serum 25(OH)D (15.5 ± 4.5 years vs 9.2 ± 5.1 years, p = 0.000). The dialysis modality differed significantly between those two groups (p = 0.002), and compared with patients on HD, patients on PD had a lower mean serum 25(OH)D concentration (14.3 ± 8.6 ng/mL vs 45.8 ± 44.0 ng/mL, p = 0.083). Among the PD patients, 90.2% had vitamin D deficiency or insufficiency (37.3% deficiency, 52.9% insufficiency). Also, although the difference was not statistically significant, the patients on continuous cycling PD (compared with those on nightly intermittent PD) had a lower mean serum 25(OH)D concentration (13.0 ± 7.5 ng/mL vs 21.1 ± 12.8 ng/mL, p = 0.142).
TABLE 2.
Characteristics of the Patients on Chronic Dialysis, by Vitamin D Status
Compared with patients having a normal serum 25(OH) D concentration, patients with a serum 25(OH)D concentration of less than 30 ng/mL had higher PTH levels (349.5 ± 318.3 pg/mL vs 142.5 ± 116.9 pg/mL, p = 0.001). We observed no significant difference in the relative dose of active vitamin D sterols between patients with a serum 25(OH)D concentration of less than 30 ng/mL and patients with a normal 25(OH)D concentration.
Associations with Serum 25(OH)D Concentration
In the univariate analysis, age was negatively correlated with serum 25(OH)D (r = -0.466, p = 0.000). Body mass index had no relationship with serum 25(OH)D. There was no correlation between serum 25(OH)D and serum 1,25 (OH)2D (r = 0.242, p = 0.064), PTH (r = -0.158, p = 0.233), calcium (r = 0.108, p = 0.415), phosphorus (r = -0.050, p = 0.706), or alkaline phosphatase (r = 0.121, p = 0.361). Serum 25(OH)D did not correlate with serum hemoglobin, albumin, or weekly total Kt/V (data not shown). There was also no correlation between serum PTH and serum 1,25 (OH)2D or the dose of active vitamin D sterols in these pediatric patients on dialysis. Serum 25(OH)D was positively correlated with renal weekly Kt/V (r = 0.385, p = 0.005; Figure 1) and CCr (r = 0.443, p = 0.001). In addition, there was a positive correlation between serum 25(OH)D and serum albumin (r = 0.297, p = 0.035) in the PD patients (Figure 2).
Figure 1.
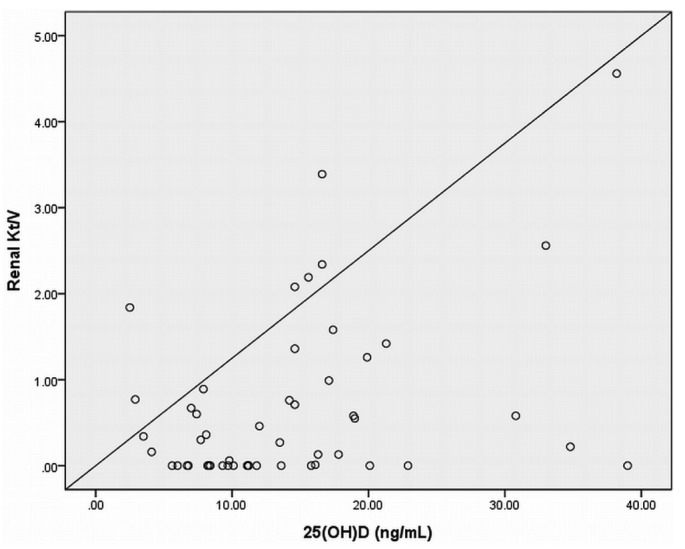
— The relationship between 25(OH)D and renal Kt/V in patients on peritoneal dialysis. Serum 25(OH)D was positively correlated with renal weekly Kt/V (r = 0.385, p = 0.005).
Figure 2.
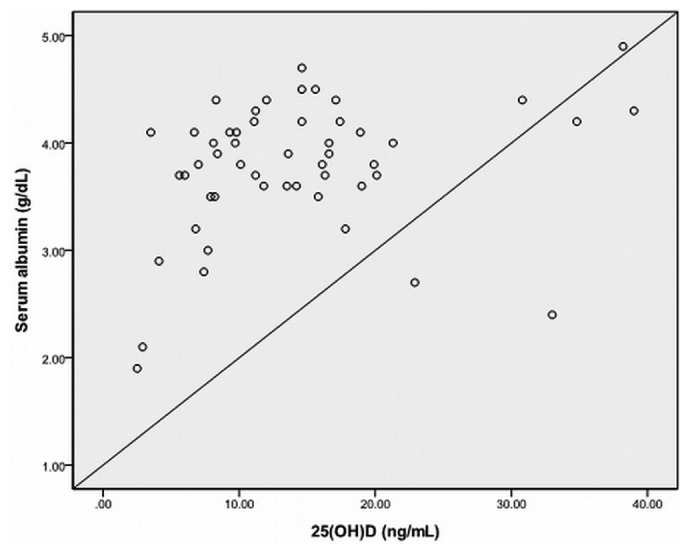
— The relationship between 25(OH)D and serum albumin in patients on peritoneal dialysis. Serum 25(OH)D and serum albumin were positively correlated (r = 0.297, p = 0.035).
Discussion
In our study cohort of pediatric patients on dialysis, the prevalence of 25(OH)D deficiency or insufficiency was 83.0%, and the mean serum 25(OH)D was 18.6 ng/mL. In adult patients on HD, the prevalence of serum 25(OH)D less than 30 ng/mL ranged from 76.1% to 97%, and mean serum 25(OH)D concentration ranged from 10.7 ng/mL to 24.4 ng/mL (16,19). One study reported that, in adult patients on PD, the prevalence of serum 25(OH)D less than 30 ng/mL was 100%, and mean serum 25(OH) D was less than 7 ng/mL (21). Most of the earlier studies on vitamin D status in CKD patients on dialysis were performed in adults, and few studies have looked at the prevalence of vitamin D deficiency in pediatric patients on dialysis. In 60 pediatric patients treated with PD, 98% had serum 25(OH)D concentrations less than 30 ng/mL (26). In pediatric renal graft recipients, 22% of non-black and 27% of black recipients were vitamin D-deficient, defined as a serum 25(OH)D of less than 10 ng/mL at transplantation, and serum 25(OH)D did not change significantly after transplantation (27).
The definition of vitamin D deficiency is not clear, and a serum 25(OH)D concentration greater than 20 ng/mL is considered indicative of vitamin D sufficiency in healthy children (11). However, because the present study includes CKD patients, a serum 25(OH)D concentration of 30 ng/mL was used as the definition of vitamin D deficiency or insufficiency according to the K/DOQI guidelines and other studies (14-17,25-27). In our study, mean serum 25(OH)D was 14.3 ng/mL in PD patients and 45.8 ng/mL in HD patients, concentrations that are relatively higher than those reported in adult patients on dialysis. We suggest that the relatively higher vitamin D stores in pediatric CKD 5 patients might be related to a better diet and more outdoor sun exposure. However, few studies have been conducted on the vitamin D status of pediatric CKD 5 patients, and further studies are necessary to clarify the differences between them and adult patients on dialysis and pediatric patients on dialysis.
In the present study, we found that patients with vitamin D deficiency or insufficiency were older than those with normal serum 25(OH)D concentrations. Although it is generally known that vitamin D deficiency is more common among elderly members of the general population, other studies of CKD patients have shown a lack of an age effect (16,19). Although we observed no sex differences in our study, others have reported that, compared with female CKD patients, male CKD patients had higher serum 25(OH)D concentrations (19). The reasons for those differences between our study and others remain unclear. We suggest that the characteristics of pediatric CKD patients on dialysis might outweigh any other effects.
We found that, compared with patients having vitamin D sufficiency, those with vitamin D insufficiency or deficiency had higher PTH levels. Another study of renal transplantation reported a relationship between serum 25(OH)D and serum PTH (22). González et al. reported that low serum 25(OH)D was associated with high serum PTH in patients with CKD stages 1-5 not requiring dialysis (16). Those results suggest that suboptimal serum concentrations of 25(OH)D may contribute to the development of secondary hyperparathyroidism in patients with CKD (16). Although hyperparathyroidism usually develops because of the impaired ability of the failing kidney in CKD patients to produce 1,25(OH)2D, less 25(OH) D available in the proximal tubular cell for conversion to 1,25(OH)2D might aggravate secondary hyperparathyroidism. Our results and previous reports might favor intervention with vitamin D (such as ergocalciferol) in CKD patients with vitamin D deficiency to prevent secondary hyperparathyroidism (16).
Although lack of sunlight and inadequate vitamin D intake are recognized as important factors contributing to vitamin D insufficiency in CKD patients, a recent study suggested that upregulation of the key catabolic enzyme 24-hydroxylase with 1,25(OH)2D therapy might be a significant mechanism contributing to vitamin D insufficiency and resistance to vitamin D therapy in CKD (28). Previous work suggested that disease models with elevated circulatory levels of FGF23, which is known as the major factor causing decreased serum 1,25(OH)2D in CKD, showed increased basal levels of renal 24-hydroxylase and FGF23, which might be responsible for this catabolic enzyme activity (28). In our study, no significant difference was observed in the relative doses of active vitamin D sterols between patients with vitamin D sufficiency and those with vitamin D deficiency or insufficiency.
We found that the mode of dialysis may be a factor contributing to vitamin D insufficiency. A possible explanation is loss of vitamin D binding protein through the peritoneal effluent in PD (29-31). In previous studies, vitamin D deficiency was proportionately much lower in HD patients than in PD patients (19,21). Vitamin D deficiency was found in 22.6% of patients on HD, but studies in PD patients showed vitamin D deficiency in more than 90% (19,21). A recent study in children on chronic PD demonstrated that peritoneal losses of vitamin D binding protein reflected both dialysate and urinary albumin losses and were associated with a longer dialysis vintage (20). In our study, although the patients using nightly intermittent PD had higher serum 25(OH)D concentrations than did patients using continuous cycling PD, the difference between the groups was nonsignificant. The effect of daily dialysis duration on vitamin D insufficiency in PD patients needs to be studied further.
We found that low levels of 25(OH)D correlated with decreased RRF in pediatric patients on PD. In pediatric and adult patients with CKD, patients with more advanced CKD (compared with those having more moderate CKD) had lower serum 25(OH)D concentrations and a significantly higher prevalence of vitamin D deficiency (14,16). Possible explanations for that finding include uremia, darker skin, and reduced sun exposure because of infirmity or dialysis modality. It is also known that suboptimal vitamin D status may be associated with the rate of progression of renal insufficiency (1-4). Few data on the relationship between vitamin D concentrations and RRF are available for patients on dialysis, and further studies are needed.
In our study, serum albumin correlated significantly with serum 25(OH)D in pediatric patients on PD. Earlier studies showed a significant, direct correlation between serum 25(OH)D and albumin in patients with CKD (16,19). Those findings suggest that inadequate nutrition and suboptimal vitamin D intake can be contributing factors in vitamin D deficiency. In addition, the earlier studies suggested that serum 25(OH)D correlates positively with serum albumin because vitamin D binding protein might typically correlate with serum albumin (29-32). Inadequate nutrition and low levels of vitamin D binding protein can play a role as contributing factors in CKD patients with low levels of serum albumin and vitamin D (16,19,32).
A study in PD patients with vitamin D deficiency showed that 25(OH)D deficiency was readily and safely corrected with one course of 50 000 IU ergocalciferol, and complaints of muscle weakness and bone pain declined (21). Previous reports suggested that PD patients are at especially high risk and might need supplementation with ergocalciferol because of ongoing loss of vitamin D binding protein through peritoneal effluent (20,21,29-32). Our results are compatible with those in previous reports and might favor supplementation with ergocalciferol in addition to active vitamin D sterols in pediatric patients on chronic dialysis.
The present study has a few limitations. There was no control group, and we did not compare vitamin D status between the general population and the CKD patients. Our study was limited to a relatively small number of patients in a single geographic location, and the study population may not be representative of all pediatric patients on chronic dialysis. Larger studies including a control group and pre-dialysis CKD patients are needed to further evaluate vitamin D deficiency or insufficiency in pediatric CKD patients.
Conclusions
In this cross-sectional study, vitamin D insufficiency or deficiency was found to be highly prevalent in pediatric dialysis patients and a possible contributor to hyperparathyroidism. More studies are needed to further evaluate the consequences of vitamin D deficiency and the impact of therapeutic interventions in pediatric patients on chronic dialysis.
Disclosures
The authors declare that there are no competing financial interests in relation to this work.
References
- 1. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 2005; 289:F8–28 [DOI] [PubMed] [Google Scholar]
- 2. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006; 116:2062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81:353–73 [DOI] [PubMed] [Google Scholar]
- 4. Vieth R. What is the optimal vitamin D status for health? Prog Biophys Mol Biol 2006; 92:26–32 [DOI] [PubMed] [Google Scholar]
- 5. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–81 [DOI] [PubMed] [Google Scholar]
- 6. Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33 [DOI] [PubMed] [Google Scholar]
- 7. Qaw F, Calverley MJ, Schroeder NJ, Trafford DJ, Makin HL, Jones G. In vivo metabolism of the vitamin D analog, dihydrotachysterol. Evidence for formation of 1α,25- and 1β,25-dihydroxy-dihydrotachysterol metabolites and studies of their biological activity. J Biol Chem 1993; 268:282–92 [PubMed] [Google Scholar]
- 8. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004; 80(Suppl):1678S–88S [DOI] [PubMed] [Google Scholar]
- 9. Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, et al. 25-Hydroxyvitamin D3-1α-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 2002; 87:2967–72 [DOI] [PubMed] [Google Scholar]
- 10. Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev 2008; 66(Suppl 2):S153–64 [DOI] [PubMed] [Google Scholar]
- 11. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008; 122:398–417 [DOI] [PubMed] [Google Scholar]
- 12. Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992; 327:1637–42 [DOI] [PubMed] [Google Scholar]
- 13. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998; 351:805–6 [DOI] [PubMed] [Google Scholar]
- 14. LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis 2005; 45:1026–33 [DOI] [PubMed] [Google Scholar]
- 15. Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A. Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol 2010; 25:2483–8 [DOI] [PubMed] [Google Scholar]
- 16. González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 2004; 24:503–10 [DOI] [PubMed] [Google Scholar]
- 17. Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr 2009; 154:906–11 [DOI] [PubMed] [Google Scholar]
- 18. Ali FN, Arguelles LM, Langman CB, Price HE. Vitamin D deficiency in children with chronic kidney disease: uncovering an epidemic. Pediatrics 2009; 123:791–6 [DOI] [PubMed] [Google Scholar]
- 19. Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int 2007; 11:315–21 [DOI] [PubMed] [Google Scholar]
- 20. Prytula A, Wells D, McLean T, Balona F, Gullett A, Knott C, et al. Urinary and dialysate losses of vitamin D-binding protein in children on chronic peritoneal dialysis. Pediatr Nephrol 2012; 27:643–9 [DOI] [PubMed] [Google Scholar]
- 21. Shah N, Bernardini J, Piraino B. Prevalence and correction of 25(OH) vitamin D deficiency in peritoneal dialysis patients. Perit Dial Int 2005; 25:362–6 [PubMed] [Google Scholar]
- 22. Sadlier DM, Magee CC. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin Transplant 2007; 21:683–8 [DOI] [PubMed] [Google Scholar]
- 23. de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 2009; 20:1805–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007; 72:1004–13 [DOI] [PubMed] [Google Scholar]
- 25. National Kidney Foundation (NKF) KDOQI clinical practice guideline for nutrition in children with CKD: 2008 Update. Recommendation 7: Bone mineral and vitamin D requirements and therapy (Web page). New York, NY: NKF; 2008. [Available at: http://www.kidney.org/professionals/KDOQI/guidelines_ped_ckd/cpr7.htm; accessed 27 April 2012] [Google Scholar]
- 26. Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 2011; 79:112–19 [DOI] [PubMed] [Google Scholar]
- 27. Tuchman S, Kalkwarf HJ, Zemel BS, Shults J, Wetzsteon RJ, Foerster D, et al. Vitamin D deficiency and parathyroid hormone levels following renal transplantation in children. Pediatr Nephrol 2010; 25:2509–16 [DOI] [PubMed] [Google Scholar]
- 28. Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int 2010; 78:463–72 [DOI] [PubMed] [Google Scholar]
- 29. Shany S, Rapoport J, Goligorsky M, Yankowitz N, Zuili I, Chaimovitz C. Losses of 1,25- and 24,25-dihydroxycholecalciferol in the peritoneal fluid of patients treated with continuous ambulatory peritoneal dialysis. Nephron 1984; 36:111–13 [DOI] [PubMed] [Google Scholar]
- 30. Joffe P, Heaf JG. Vitamin D and vitamin-D-binding protein kinetics in patients treated with continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1989; 9:281–4 [PubMed] [Google Scholar]
- 31. Gokal R, Ramos JM, Ellis HA, Parkinson I, Sweetman V, Dewar J, et al. Histological renal osteodystrophy, and 25 hydroxycholecalciferol and aluminum levels in patients on continuous ambulatory peritoneal dialysis. Kidney Int 1983; 23:15–21 [DOI] [PubMed] [Google Scholar]
- 32. Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin). Endocr Rev 1989; 10:294–307 [DOI] [PubMed] [Google Scholar]


