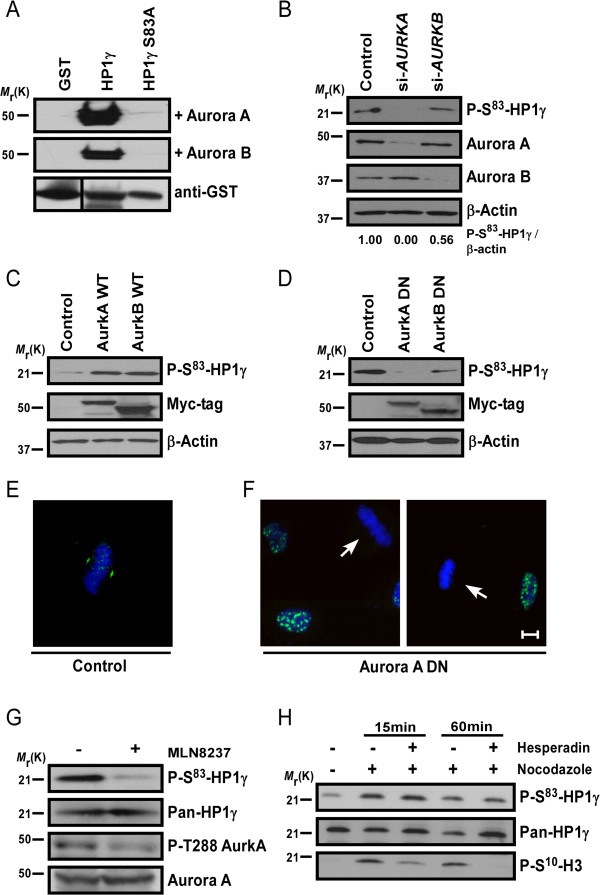
Figure 4.
Aurora A phosphorylates Ser83-HP1γ in G2/M. (A) Aurora kinases phosphorylate Ser83 in vitro. In vitro kinase assays were performed on GST fusion proteins, which demonstrate that wild type, not S83A-HP1γ mutant, is phosphorylated by Aurora kinases. (B) Aurora A siRNA reduces P-Ser83-HP1γ. Aurora A siRNA significantly reduced P-Ser83-HP1γ, whereas Aurora B siRNA only slightly reduced P-Ser83-HP1γ (top). Aurora A (AURKA) and Aurora B (AURKB) were effectively knocked-down (middle panels). Relative intensities were calculated as P-Ser83-HP1γ/β-actin ratios. (C) Wild type Aurora kinases increase P-Ser83-HP1γ. CHO cells, with low basal P-Ser83-HP1γ, demonstrated increased P-Ser83-HP1γ (top) upon transfection of Aurora kinases (Myc-tag; middle). (D) Aurora A-dominant negative (DN) reduces P-Ser83-HP1γ. P-Ser83-HP1γ (top) was significantly reduced with Aurora A-DN in BxPC3, epithelial cells with high basal P-Ser83-HP1γ. Aurora B-DN also reduced P-Ser83-HP1γ, although still detected. Aurora-DN levels are shown by Myc-tag. β-actin serves as loading control (B, C, D; bottom). (E,F) Aurora A-DN abolishes mitotic P-Ser83-HP1γ. Representative images of overlays with DAPI counterstain are shown for P-Ser83-HP1γ (green) with control (E) or Aurora A-DN (F). Typical P-Ser83-HP1γ localization was still observed in interphase with Aurora A-DN, but disrupted in metaphase (arrows). Scale bar represents 10 μM. (G,H). Pharmacological inhibition of Aurora A, but not Aurora B, inhibits P-Ser83-HP1γ. Aurora A inhibition with MLN8237 was confirmed by loss of activated P-Thr288 relative to total Aurora A (G, lower panels). P-Ser83-HP1γ was significantly reduced with MLN8237, without affecting pan-HP1γ (G, upper panels). Conversely, Aurora B inhibition by hesperidin did not reduce P-Ser83-HP1γ (H, top). Aurora B inhibition was confirmed by P-Ser10-H3, a well-known Aurora B target (H, bottom). CHO, Chinese hamster ovary; DAPI, 4',6-diamidino-2-phenylindole; DN, dominant negative; GST, glutathione S-transferase; P-Ser10-H3, phosphorylation of histone H3 at serine 10; P-Ser83-HP1γ, phosphorylation of HP1γ at serine 83; P-Thr288, phosphorylation of Aurora A at threonine 288; Ser83, serine 83.