Abstract
We studied risky decision making (RDM) in 8 healthy adolescents (TC) and 11 adolescents with mild to moderate traumatic brain injuries (TBI) using the Balloon Analog Risk Task (BART) and fMRI. Participants inflated simulated balloons (with more points awarded to bigger balloons), which might burst at any time. Increasing brain activation levels were associated with increasing balloon size in a largely bilateral network, including cerebellar, inferior parietal, limbic, and frontal areas. Both groups performed similarly and activated similar networks.
We face risk while making decisions in virtually every aspect of our lives. Decisions may be financially, physically, or socially risky in their respective realms because they trade off potential rewards for potential loss, harm, or danger in the presence of uncertainty. This issue is of particular interest in adolescence, a developmental period frequently associated with an increased potential for risk taking compared to other life periods. The difficulty that teenagers have in making prudent choices about risk may be further exacerbated following a traumatic brain injury (TBI). Deficits in higher-order cognitive processes commonly observed in pediatric TBI are often characterized as deficits in executive functions (EF), which encompass attention, cognitive control, inhibition, working memory, and decision making (see Tlustos et al., 2011 for citation of additional review studies). However, little extant research has focused on risky decision making (RDM) and its neural underpinnings in children following TBI because there are few standardized measures for this construct suitable for younger populations. In adults, numerous neuroimaging studies have shown that a network of brain areas, including the midbrain, the striatum, the amygdala, the insula, the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), medial prefrontal cortex, and orbitofrontal cortex (OFC), mediates different aspects of risky decision making. Only a small number of studies have sought to characterize how areas in this network operate in childhood and adolescence (e.g., see Van Leijenhorst et al., 2010 for review). From these studies, it appears that the ventral striatum, the insula, ACC, OFC, and cingulate gyrus bilaterally are significant components of a neural network in adolescence important for reward processing and cognitive control, which are two critical aspects of risky decision making, but that across studies adolescents have shown both higher and lower levels of activation in parts of the relevant neural network relative to both adults and younger children.
This paper reports the results of a small preliminary study of the neural correlates of risky decision making in adolescents following TBI using the Balloon Analog Risk Task (BART) (Lejuez et al., 2002, Rao et al., 2008). On each trial, participants pressed keys to inflate a virtual balloon on a computer screen. Bigger balloons garnered more points and thus could serve as an index of perceived risk/reward. Participants decided prior to each pump whether to continue to pump or collect the current point total and move on to the next trial. When a balloon was inflated beyond the burst size (randomly selected from trial to trial and unknown to the participant), the participant lost and had to proceed to the next trial. Healthy adults showed increasing levels of activation in a mesolimbic-frontal network (MLF), including the midbrain, the striatum, the insula, dorsolateral prefrontal cortex, and anterior cingulate / medial frontal cortex, when they chose to inflate the balloon to larger sizes (Rao et al., 2008). In contrast to the risk tasks used in the earlier fMRI studies of the adolescent brain, the BART does not present a static choice between options with known probability of payoffs and reward magnitude. Instead, it involves sequential risk-taking with feedback and has the advantage of being dynamic, incremental, and engaging for participants in this age range. Using fMRI, we have previously observed increased brain activation associated with cognitive control in adolescents with TBI relative to a cohort of age and sex-matched healthy controls (Tlustos et al., 2011). This study examines RDM via BART in the same population using fMRI as well.
METHOD
Participants
Details of the participant source and selection criteria are as in Tlustos and colleagues (2011). All adolescents with TBI were required to be ≥ 12 months post injury and were required to have sustained a TBI that required overnight hospitalization. These participants with TBI were identified through their participation in ongoing behavioral intervention studies. Both the intervention project and the current imaging study were approved by the Institutional Review Board. All participants with TBI had either initial Glasgow Coma Scale (GCS) scores in the 10–11 range (n=3) or initial GCS scores of 13–15 accompanied by positive findings on CT or MRI (n=8). The average time since their injury was 1.85 years (SD = .45). A comparison cohort of typically-developing adolescents matched with the TBI group on sex, handedness, and race/ethnicity, and maternal education was recruited as controls (TC). A total of 19 children who completed informed consent to participate in the study yielded usable fMRI data. These 19 participants included 11 adolescents (mean age = 15.7, range = 14.3 to 17.0, s.d. = 0.9) with TBI (with lowest GCS ranging from 10 to 15) and 8 typically developing adolescents (TC) (mean age = 16.3, range = 14.3 to 17.5, s.d. = 1.0). Adolescents completed a brief neuropsychological battery after giving informed consent. The measures included Peabody Picture Vocabulary Test-Fourth Edition (PPVT-IV; mean = 98 for TBI and 109 for TC, p = 0.07), the Wide Range Achievement Test-Fourth Edition (WRAT-4; mean = 99 for TBI and 116 for TC, p = 0.05), the Working Memory (WM) and Processing Speed (PS) Indices from the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV, mean WMI = 99 for TBI and 107 for TC, p > 0.15, and mean PSI = 108 for TBI and 104 for TC, p > 0.65), and the Delis-Kaplan Executive Function System Color Word Interference Test (mean standard score = 10.3 for TBI and 9.4 for TC, p > .25). Parents and adolescents completed the Behavior Rating Inventory of Executive Function (BRIEF) and BRIEF-self report (BRIEF-SR) respectively (mean BRIEF Global Executive Composite (GEC)T-score = 57 for TBI and 44 for TC, p = 0.04, and mean BRIEF-SR GEC = 55 for TBI and 52 for TC, p > 0.46). See Tlustos and colleagues (2011) for more details regarding the measures and instruments used.
Stimuli and Behavioral Tasks used for fMRI
In the variant of the BART used here (cf. Lejuez et al., 2002; Rao et al., 2008), participants were asked to play a computer game and try to score as many points as possible. On each trial of the game, an orange balloon was presented in the center of the computer screen in an un-inflated initial size. Participants were told that they could press one button (pump) to sequentially inflate the balloon by a constant amount (i.e., two points) on each pump, or press another button once to cash out and collect the points accrued on the current trial (collect), presumably because they reached some internal threshold of risk tolerance. If, however, a balloon was pumped beyond its burst point on the current trial, the computer screen would display an exploded balloon, and all points on the current trial would be lost. When a balloon exploded or the participant pressed the collect button, the screen would display the score accrued on the current trial for 2 seconds, followed by the display of the total score accrued so far for 4 seconds. At this point, an un-inflated balloon would be presented and the next trial ensued. Twenty trials were presented for each scan. Participants were told that the explosion point would vary unpredictably from trial to trial during each scan, and they were to remain still if they finished before the scan ended (and all of them did, with the MR data acquired after they finished the task excluded from analyses). The set of burst points used for all participants followed the same semi-random, pre-determined order. The minimum and maximum allowable number of pumps for any balloon was 2 and 64 respectively. Participants were given one example during a pre-scan training session, and all indicated understanding of the instructions. No further training was provided. Participants were also encouraged to try their best to obtain the highest score.
All MR scanning procedures and post-processing steps were as in Tlustos and colleagues (2011). Reconstructed EPI data were co-registered and transformed into Talairach coordinates. FMRI data were included for a particular participant only if fewer than 10% of the MR volumes across time points showed excessive movement (i.e., a displacement of 0.75 voxel in any 3-D direction) from the reference volume for that scanned task. Participants in the TBI and TC group did not differ in mean displacement, median displacement, and proportion of MR volumes across time points where displacement exceeded 0.75 voxel, all ps > 0.11. For each participant, a reference function for risk level was defined by convolving the balloon size, which increased linearly over each balloon pump on each trial up to the point prior to collect or explosion, with a canonical hemodynamic response (HDR) to examine the brain activations that co-varied with voluntary risk taking (cf. Rao et al., 2008). Because of the limited scan duration and low frequency associated with other event types of interest (e.g., explosion and collect) those additional event types were not modeled. For each participant, a Pearson's partial correlation analysis was computed on a voxel-wise basis between EPI data and the task reference function using the motion correction parameter as a covariate. A 6s delay was applied to the reference function to allow for the canonical hemodynamic response to peak. Correlation coefficients were transformed into z-score map and t-tests were performed at the group level (voxelwise Bonferroni-corrected FWE p, 2-tailed, = 0.05, clustering threshold = 50, 6mm FWHM post-processing filtering). Based on Ledberg and colleagues (1998), Monte Carlo simulations were used to estimate corrected p-values to explore potential group-related differences, in which the spatial autocorrelations present in the fit residuals were used to estimate the intrinsic smoothness in the data. “Null” activation maps were generated from spatially auto-correlated Gaussian noise generated using the previously found smoothness estimates and post-processing parameters (e.g. threshold intensity, cluster size, and exogenous spatial filtering). The simulations were repeated, and the corrected p-values estimated by computing the proportion of null maps with spurious activated clusters detected. Monte-Carlo simulation was performed to assure p < 0.001 after adjusting for multiple comparisons.
RESULTS
Performance data for the BART were recorded during the fMRI scan. Following Lejuez and colleagues (2002), the adjusted number of pumps (i.e., the average number of pumps attempted per trial excluding all trials for which the balloon exploded) provided a measure of the risk tolerance threshold for individual participants. There were no significant differences between groups on the BART performance variables including the total score (501 vs. 497 for TBI and TC respectively, p = 0.88), percentage of exploded balloons (38% vs. 38%), and average adjusted number of pumps (20.6 vs. 20.6). However, there was substantial variability within each group. For example, average adjusted number of pumps ranged from 13.4 to 28.3 in the TBI group and from 14.1 to 24.4 in the TC group. The adjusted number of pumps was significantly and positively correlated with the total score achieved (r = 0.8, p < 0.0001 and percent exploded (r = 0.83, p < 0.0001), which were themselves not significantly correlated (r = 0.34, p = 0.143). A preliminary principle component analysis involving the 3 BART variables showed that over 75% of the variance was accounted for by the 1st component, on which the adjusted number of pumps had a 0.998 factor loading. All of these results regarding BART were unchanged when co-varying for group status. We therefore focused on the adjusted number of pumps as the primary measure of risk tolerance in subsequent analyses.
BART fMRI results
Correlation Pattern Overall
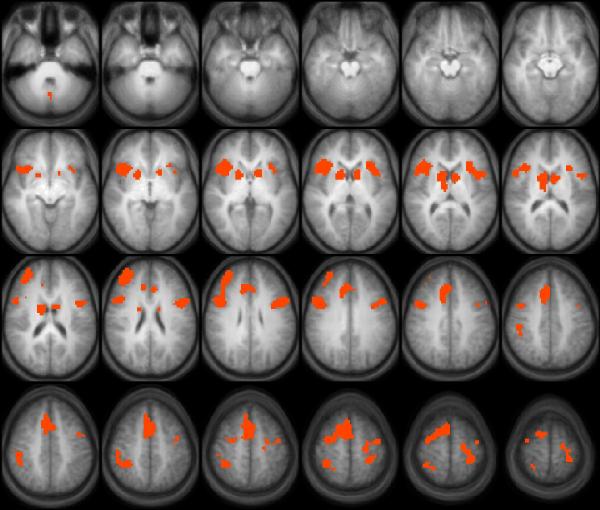
Figure 1 presents the composite p-map of brain regions with activation levels that significantly correlated with the adjusted number of total pumps and corresponded to increasing balloon size (cf. Rao et al., 2008, voluntary risk condition) across the entire sample of 19 participants. This network included several areas that have been implicated in previous fMRI studies of risk taking and also reported in Rao and colleagues (2008), such as bilateral striatum (e.g., sample foci in (17, 9, 3) and (−14, 9, 3) respectively), midline thalamic regions (8, −9, 11), bilateral insula (BA 13; sample foci in (35, 15, −1) and (-32, 21, 7) respectively), the medial frontal cortex (BA 8; sample focus in (4, 18, 43)), anterior cingulate cortex (ACC / dACC, BA 32; sample foci in (−5, 27, 27) and (8, 18, 39)), a more anterior and right-lateralized portion (BA10; sample focus in (29, 48, 23)) as well as a bilateral portion of the dorsolateral prefrontal cortex (DLPFC, BA 9; sample foci in (44, 12, 23) and (−47, 9, 27) respectively), bilateral inferior parietal lobule (IPL, BA 40; sample foci in (44, −36, 43) and (−29, −36, 55) respectively), and additional areas in frontal cortex (BA 4, sample focus in (−40, −9, 55) and BA 6, sample focus in (16, 6, 55), respectively). A quick comparison between Figure 1 and Table 2 of Rao and colleagues (2008) revealed very significant overlap between the two maps. When the WRAT and BRIEF-PR, which showed significant group differences behaviorally, were included as additional covariates of no interest, no foci survived the thresholds established in the Monte Carlo simulations for between-group comparisons. A specific test for between-group differences confined to the OFC, the ACC or the bilateral striatum did not find any signs of a significant difference (all ps > 0.5).
Figure 1.
DISCUSSION
We observed increasing levels of brain activation in a set of brain foci as the adolescent participants pumped up the balloons to seek larger and larger rewards. Consistent with earlier findings in normal adults (cf. Rao et al., 2008), this network included the striatum bilaterally, thalamic regions, dorsal ACC (BA 32), the insula bilaterally, and other areas of the prefrontal cortex (BA 9, 10, 44, & 47). Addition areas in the frontal cortex, which are traditionally thought of as serving a role oriented more towards sensory-motor processing (e.g., BA 4 & 6), have not been reported to be involved in Rao and colleagues (2008). However, some of these differences are likely due to procedural factors (e.g., Rao and colleagues (2008) imposed a random “rest” period of 1.5 to 2.5 s after each balloon pump and we did not, thereby increasing the amount of sensori-motor processing related to pumps). Using Monte Carlo simulations, we did not detect any foci that showed differences between the groups at conventional levels of significance, after controlling for the contribution of covariates. With respect to the functional role played by these brain areas, we have previously reported that adolescents with mild to moderate TBI showed higher levels of activation than healthy adolescents in several cortical regions related to cognitive control in a Counting Stroop task relative to non-injured controls despite comparable levels of performance (Tlustos et al., 2011). Those regions included frontal and parietal regions such as BA 8 (with centroid coordinates at (4,21,47)) in the midline frontal area, ACC (4, 24, 39), and Inferior Parietal Lobule (BA 40) (40, −33, 35), which are quite close to the ones identified in Figure 1, and may be expected to play a role of cognitive control in the BART.
Our current adaptation of the BART for fMRI is relatively fast-paced and of short duration, which is a distinct advantage for our younger participants for task engagement. On the other hand, our analyses are focused only on those periods during each trial before the occurrence of any events related to feedback or end-of-trial (i.e., participants chose to collect or the balloon exploded) for several reasons. First, the fMRI signals from these events of interest (e.g., outcome anticipation and processing, motor response preparation and maintenance, etc.) occurred very closely in time so it is difficult to resolve these signals individually. Second, certain events are naturally confounded in the BART (e.g., higher risk confounded with higher reward) that makes it difficult to distinguish the separate contribution of each. The limitations posed by these challenges need to be taken into account in future task modifications.
The small sample size and the issue of insufficient statistical power clearly limit the generalizability of the present results to the broader populations, particularly with respect to any failure to find a difference (either behaviorally or in fMRI) between groups, or whether or not we confine our analysis to the broader ROIs or limit them to specific brain regions such as the ACC, striatum or the OFC. Even though children with severe TBI often display poorer performance than less-severely injured or healthy control children across EF domains, participants with less severe injuries can perform relatively normally (e.g., Catroppa & Anderson, 2003). We did not have any participants with severe TBI in the current study, so it may not be surprising that we did not find any behavioral differences between groups on the BART. It is noteworthy, however, that differences in other measures such as the BRIEF, WRAT and PPVT did reveal differences that approached or reached statistical significance. Second, with the exception of working memory index, performance on the BART was largely unrelated to other neuropsychological or cognitive measures in the current sample. Bornovalova and colleagues (2009; also see Lejuez et al., 2002) also reported age, gender, and family income as unrelated to any of the BART variables. Higher working memory load have been observed to lead to greater discounting of delayed future rewards, greater preference for immediate rewards, and increased signs of impulsivity (Hinson, Jameson & Whitney, 2003). By implication, those with a lower working memory index in the current study might have preferred then to pump less and opted for the immediate reward points in hand. Taken together, these observations provide no support yet for the claim that adolescents with TBI perform differently on RDM tasks (at least for the BART) than their non-injured counterparts.
Our neuroimaging findings related to anticipation of outcome and rewards are consistent with those reported by Van Leijenhorst and colleagues (2010b), who had healthy participants (age 10–12, 14–15, or 18–23) play a slot machine game. Van Leijenhorst and colleagues were interested in the differences in brain activation when participants either waited with greater anticipation and excitement to see if they won (cheeries-cheeries-???) or if they knew at this point that the trial would be a non-reward trial (cherries-pears-???). Van Leijenhorst and colleagues reported activation related to outcome anticipation in bilateral insula, striatum, dorsal cingulate cortex, parietal areas, and various areas in the frontal lobes in adolescents (age 10–12 and 14–15). They also reported that such differential activation was present in the striatum but not in the anterior insula for the adults. These results highlight the importance of the striatum, the insula, and parts of the medial prefrontal cortex in mediating outcome and reward anticipation in RDM. Importantly, while these areas are also implicated in the current study, there were no clear differences in the magnitude of the correlation across groups in these areas. Whether the basic appreciation and processing of risk and reward in the BART is different in adolescents with mild/moderate TBI and their non-injured counterparts remains to be determined in future, larger studies with more statistical power to detect potential group differences.
Acknowledgments
This work was supported in part by 1) NIH grant RO1-MH073764 from the National Institute of Mental Health (to S. Wade); 2) H133G050239 from the National Institute on Disability and Rehabilitation Research in the Department of Education (to S. Wade), and 3) EMS/Trauma grant from the Ohio Department of Public Safety (to S. Wade).
References
- Bornovalova MA, Cashman-Rolls A, O'Donnell JM, Ettinger K, Richards JB, deWit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacology, Biochemistry, & Behavior. 2009;93:258–262. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. Children's attentional skills 2 years post-traumatic brain injury. Developmental Neuropsychology. 2003;23:359–373. doi: 10.1207/S15326942DN2303_3. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the balloon analog risk task (BART) Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlustos SJ, Chiu C-YP, Walz NC, Holland SK, Bernard L, Wade SL. Neural correlates of interference control in adolescents with traumatic brain injury: FMRI study of the counting Stroop task. Journal of the International Neuropsychological Society. 2011;17:181–189. doi: 10.1017/S1355617710001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]