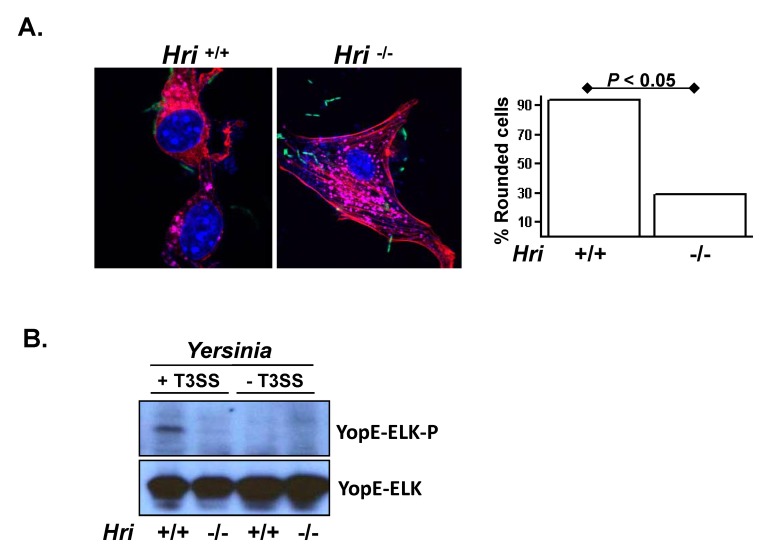
Figure 2. HRI facilitates the translocation of Yersinia virulence factors into the cytosol.
(A) Hri +/+ and -/- mouse embryonic fibroblasts (MEFs) were infected with a GFP-expressing strain Y. pseudotuberculosis for 2 hrs and then stained for nuclei, vacuoles, and actin (purple, pink, and red, respectively). Plotted is the percentage of rounded cells (resembling the cell shown in the left panel) of at least 5 microscopic fields of approximately 100 total cells. P value calculated using student t test. (B) MEFs were infected with a Y. pseudotuberculosis strain deleted for all six Yop effectors and possessing either an intact or a translocation-defective type 3 secretion (T3S) system and harboring a plasmid encoding a YopE-Elk translocation reporter. After 1 hr of infection the resulting whole cell lysates were examined by western analysis using antibodies specific for the Elk epitope tag either in its phosphorylated (YopE-Elk-P) or unphosphorylated (YopE-Elk) forms. YopE-Elk becomes phosphorylated exclusively within the eukaryotic cytosol and therefore the phosphorylation of the Elk epitope is readout for T3S-mediated translocation. For presentation purposes the lanes of a single blot were rearranged.