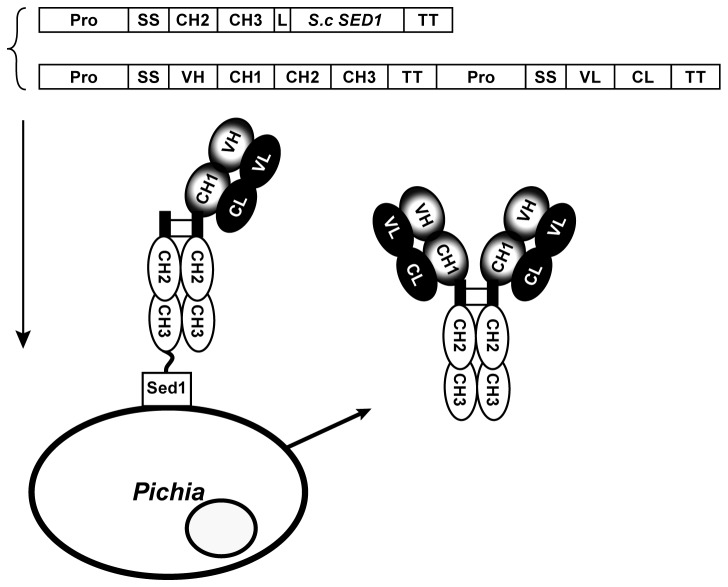
Figure 1. A schematic representation of the Fc-Sed1p antibody display system.
The DNA sequence of the hinge region along with the CH2 and CH3 domains comprising the IgG Fc fragment are fused through a flexible linker to a cell wall anchoring partner, in this case the S. cerevisiae GPI anchor Sed1p is used. When co-expressed in the same host with a secretable full length IgG molecule, the Fc portion of the anchored fusion (the bait) heterodimerizes in the ER with the Fc region of the IgG molecule, forming two disulfide bridges. Since the CH1 domain of the heavy chain can still pair with the CL domain of the secreted light chain, this tripartite complex results in surface display of the monovalent (H+L) IgG molecule. Meanwhile the assembly of soluble full length IgG occurs with equal probability resulting in secretion of the bivalent (H2+L2) in the culture medium.