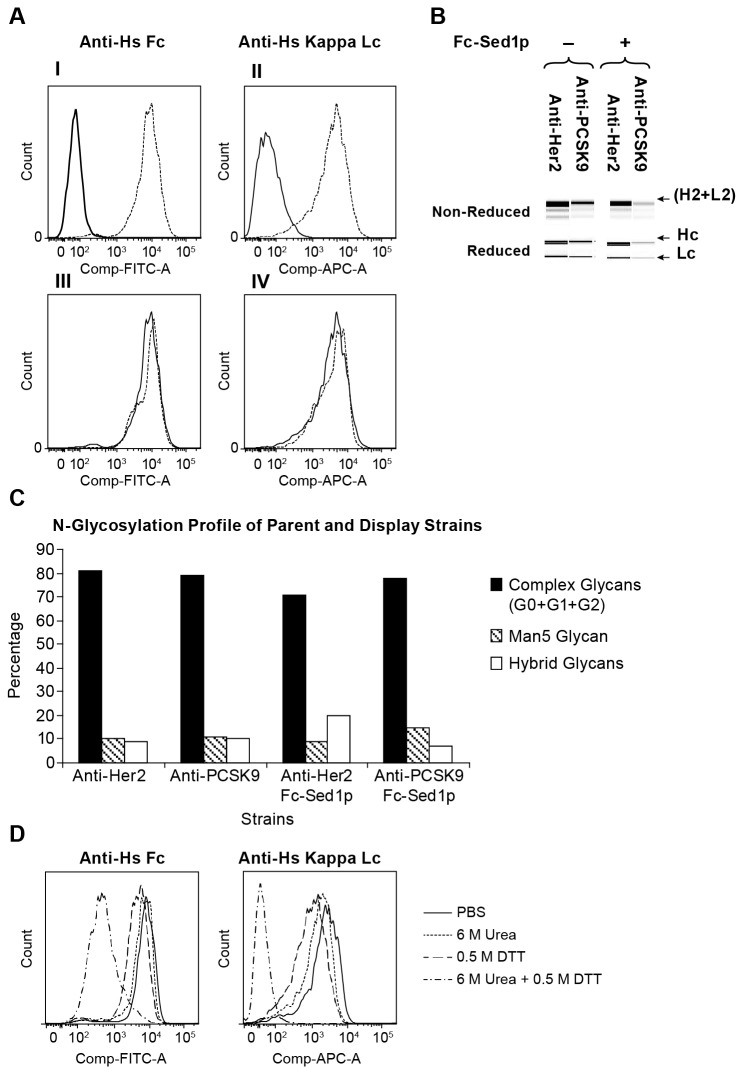
Figure 2. Efficiency of the surface anchored Fc bait (Fc-Sed1p) in capturing monovalent IgG molecules on the cell wall of Glyco-engineered Pichia pastoris.
A) (I) Strain expressing soluble anti-PCSK9 antibody (solid histogram) and anti-PCSK9 strain co-expressing Fc-Sed1p fusion (dotted) were incubated with DyeLight 488 anti-Fc antibody and florescence intensities were assayed by flow cytometry (II). Same strains as in (I) were incubated with APC-conjugated anti-Kappa antibodies to detect light chain on cell surface (III). Flow cytometry was used to compare strain co-expressing anti-PCSK9 and Fc-Sed1p anchor (solid) against strain co-expressing anti-Her2 and Fc-Sed1p (dotted) for binding to DyeLight 488 anti-Fc or (IV) APC-anti Kappa. B) Detection of co-secreted full length IgG in antibody producing strains with and without Fc-Sed1p. Culture medium was passed through a protein A column, IgGs were eluted and protein was resolved in a native or reduced form through Protein Chip analysis C) Bar graph of percentage human N-glycosylation abundances of protein A purified mAbs isolated in B. D) Cells co-expressing anti-PCSK9 and Fc-Sed1p fusion were induced and incubated for 10 minutes at room temperature in 1XPBS buffer (solid); 1XPBS buffer containing 6 M Urea (dotted); 1XPBS buffer containing 0.5 M DTT (dashed); or 1XPBS containing 6 M Urea and 0.5 M DTT (complex). Cells were washed and labeled with anti-human Fc (I) (DyeLight 488) and anti-human Kappa Lc (II) (APC). Fluorescence intensities were analyzed by flow cytometry.