Abstract
The S100 protein family consists of 24 members functionally distributed into three main subgroups: those that only exert intracellular regulatory effects, those with intracellular and extracellular functions and those which mainly exert extracellular regulatory effects. S100 proteins are only expressed in vertebrates and show cell-specific expression patterns. In some instances, a particular S100 protein can be induced in pathological circumstances in a cell type that does not express it in normal physiological conditions. Within cells, S100 proteins are involved in aspects of regulation of proliferation, differentiation, apoptosis, Ca2+ homeostasis, energy metabolism, inflammation and migration/invasion through interactions with a variety of target proteins including enzymes, cytoskeletal subunits, receptors, transcription factors and nucleic acids. Some S100 proteins are secreted or released and regulate cell functions in an autocrine and paracrine manner via activation of surface receptors (e.g. the receptor for advanced glycation end-products and toll-like receptor 4), G-protein-coupled receptors, scavenger receptors, or heparan sulfate proteoglycans and N-glycans. Extracellular S100A4 and S100B also interact with epidermal growth factor and basic fibroblast growth factor, respectively, thereby enhancing the activity of the corresponding receptors. Thus, extracellular S100 proteins exert regulatory activities on monocytes/macrophages/microglia, neutrophils, lymphocytes, mast cells, articular chondrocytes, endothelial and vascular smooth muscle cells, neurons, astrocytes, Schwann cells, epithelial cells, myoblasts and cardiomyocytes, thereby participating in innate and adaptive immune responses, cell migration and chemotaxis, tissue development and repair, and leukocyte and tumor cell invasion.
Keywords: S100 protein, calcium binding, calcium homeostasis, DAMPs, inflammation, cancer, tissue repair/regeneration, RAGE, TLRs, signaling pathways
INTRODUCTION
Ca2+-binding proteins have evolved from a common ancestor to regulate intracellular Ca2+ levels and numerous Ca2+-signaling pathways [1,2]. Those that regulate Ca2+ levels are typically membrane proteins which have evolved to pump Ca2+ to the outside the cell, or towards intracellular Ca2+ stores to maintain low cytosolic free Ca2+ concentrations under resting conditions (~100 nM). This is a physiological requirement necessary to avoid Ca2+ precipitation and/or excess Ca2+ signal activity. For example, the Ca2+-ATPase associated with the endoplasmic reticulum constitutes a Ca2+ reserve that can be released into the cytoplasm as necessary for specific cellular functions. Some other Ca2+-binding proteins (e.g., calsequestrin and calretinin) are characterized by a low Ca2+-binding affinity but high Ca2+-binding capacity, due to their high levels and location within Ca2+ stores. Here they bind the ion, but make it available for release when required. Other Ca2+-binding proteins, mostly cytoplasmic, either buffer Ca2+ during the course of Ca2+ transients as a result of their high Ca2+-binding affinity (e.g., parvalbumin and S100G) or function as transducers of the Ca2+ signal. This latter group represents a large fraction of Ca2+-binding proteins, which when bound to Ca2+, interact with other protein targets to regulate a large number of cellular functions. Calmodulin, troponin-C and most S100 proteins are considered Ca2+-signaling proteins and all have the conserved calcium-binding motif termed the EF-hand.
Contrary to calmodulin and troponin-C, whose activities are restricted to the intracellular milieu, several S100 proteins act as intracellular regulators and as extracellular signaling proteins and may be secreted and/or released to regulate activities of target cells in a paracrine and autocrine manner. Importantly, S100 proteins are expressed exclusively in vertebrates, and exhibit somewhat cell-specific distribution [3]. Of the 24 human S100 genes, 19 (S100 proteins, group A) are located within chromosome 1q21 [4]. Other gene locations include S100A11P, which maps to chromosome 7q22-q3, S100B, which maps to chromosome 21q22, S100G, which maps to chromo-some Xp22, S100P, which maps to chromosome 4p16, and S100Z, which maps to chromosome 5q13 [4].
Within cells, S100 proteins have been involved in the regulation of proliferation, differentiation, apoptosis, Ca2+ homeostasis, energy metabolism, inflammation and migration/invasion through interactions with a variety of target proteins including enzymes, cytoskeletal subunits, receptors, transcription factors and nucleic acids. Extracellular S100 proteins act in an autocrine and paracrine manner via activation of surface receptors, G-protein-coupled receptors, scavenger receptors, or heparan sulfate proteoglycans and N-glycans. As extracellular signals, S100 proteins have been shown to regulate cell proliferation, differentiation, survival and migration in normal and pathological conditions, inflammation and tissue repair, and/or to exert antimicrobial activity. Certain S100 proteins are also found in serum and other biological fluids during the course of pathological conditions and are used as disease markers.
Numerous S100 genes are induced in a somewhat cell-specific manner, by appropriate growth factors, cytokines and toll-like receptor (TLR) ligands. In these circumstances, they are generally secreted, and may function as extracellular alarmins or damage-associated molecular pattern factors that principally mediate functions of the innate and adaptive immune systems, stimulate cancer cell locomotion and/or participate in tissue repair [5-13]. Increased expression of certain S100 proteins may also enhance intracellular regulatory activities, as outlined below. Lastly, in particular cases, induction of expression of an otherwise repressed S100 gene may be functionally linked to the cell's response to an intervening event, particularly to stress. For example, S100B is not expressed in cardiomyocytes in normal physiological conditions, but is induced in cardiomyocytes surviving an infarct, and can limit the hypertrophic response by inhibiting expression of α-actin and β-myosin [14]. S100A8 and S100A9 expression is upregulated in numerous cell types by oxidative stress, corticosteroids and by particular cytokines and growth factors [9]. However, information about the regulation of expression of most S100 proteins in normal and pathological conditions is fragmentary.
EF-HAND MOTIFS OF S100 PROTEINS
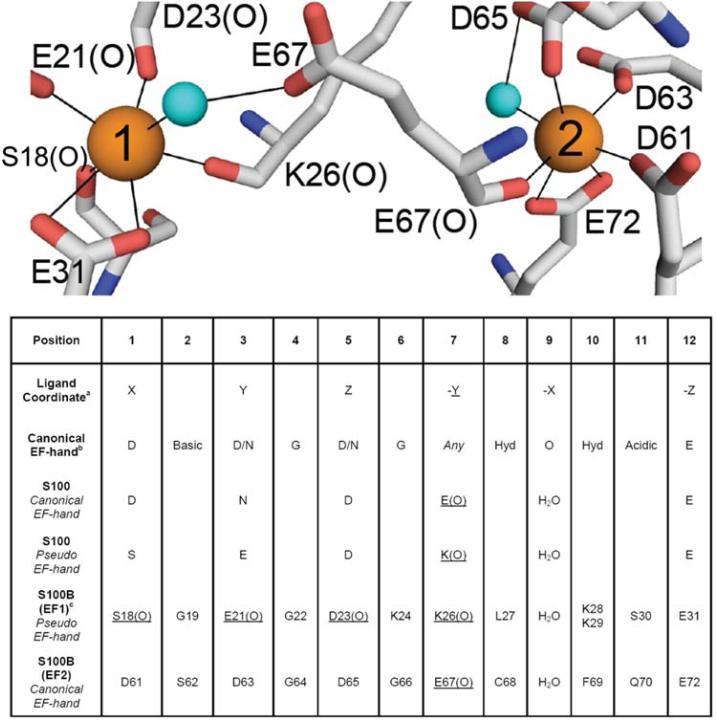
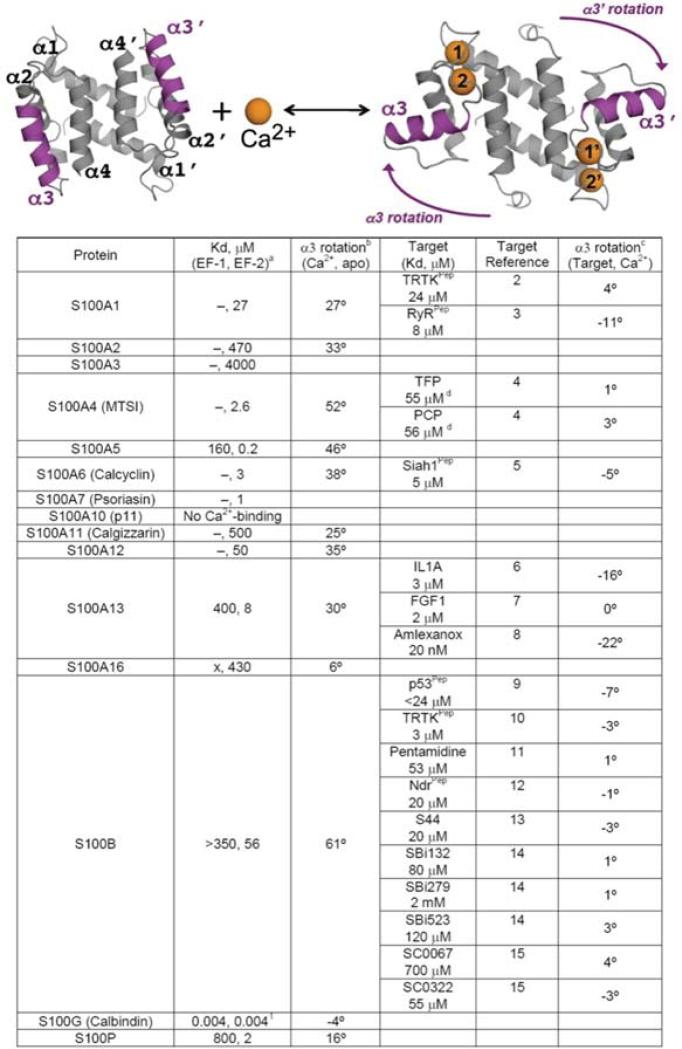
Since the E- and F-helices in the helix-loop-helix calcium-binding motif of parvalbumin were characterized by Kretsinger and colleagues [15], there have been over 650 crystal and nuclear magnetic resonance (NMR) structures of EF-hand Ca2+-binding proteins deposited into the protein data bank. Typically, EF-hand Ca2+-binding motifs are arranged in pairs of EF-hands held together by a very short anti-parallel β-strand and numerous hydrophobic interactions between the four helices. The canonical EF-hand has 12-residues with six or seven backbone or sidechain oxygen ligands utilizing residues in positions 1, 3, 5, 7, and 12 (bidentate) of the helix-loop-helix calcium-binding domain (Fig. 1) [16]. S100 family proteins, on the other hand, are a unique set of EF-hand family members since one of the EF-hand motifs in the pair (termed the pseudo- or S100-hand) has 14 rather than 12 residues, and several of the ligands bind to Ca2+ include backbone carbonyl oxygen atoms rather than oxygen atoms from sidechain Asn, Asp, Gln, or Glu residues [17]. Likewise, the S100 proteins are dimeric, which does not allow for the movement of the exiting helix (helices 4, 4′) upon Ca2+-binding as is found for other EF-hand proteins (i.e. calmodulin, troponin-C, etc.) [18]. Instead, it is the entering helix (helices 3, 3′), which rotates as much as 90 degrees upon binding Ca2+ to expose a hydrophobic patch as necessary for interacting with its specific protein targets (Fig. 2) [19].
Fig. (1).
Coordinating residues for the canonical (site 2) and S100 EF-hand (site 1) for the S100 protein, S100B. In the table below the residues typically at each position of the EF-hand are illustrated. It should be noted that the S100 EF-hand has 14 rather than 12 residues.
Fig. (2).
Ribbon diagram illustrating the rotation of helix 3 upon the addition of calcium and a table listing the degree of movement upon calcium and target protein and/or drug binding. The dissociation constants are from [8].
A question that has arisen is how can a cell have a large number of intracellular Ca2+-binding proteins at high concentration without sequestering too much free Ca2+ ions, which is needed for signaling biological events, in the nM to very low μM range range (i.e. 100 nM to 2 μM)? Although the mechanistic details for this process are still being characterized, it is usually the case that, EF-hand binding proteins, including many S100 proteins (i.e. S100A1, S100B, and others), do not bind Ca2+ very tightly in the absence of their biological target (KD >10 μM; Fig. 2) [20]. It is the protein-target interaction itself that is necessary to allosterically regulate the complex, so that the EF-hand binding protein is then able to appreciably bind Ca2+ at physiologically relevant free Ca2+ ion concentrations inside the cell to signal for a functional response [20,21]. However S100A10 does not conform to other family members because it lacks a functional EF-hand Ca2+-binding domain, so that its target-protein interactions are Ca2+-independent [22,23]. It is also clear that some peptide targets show this trend, but generally they are not sufficiently intact to induce the same effects typical of the full-length proteins. Thus, target peptides derived from the ryanodine receptor (RyR) lower the dissociation constant of S100A1 by about a factor of 10; whereas, full-length RyR enabled S100A1 to interact with Ca2+ at 100 nM free Ca2+ concentration. This represents an over 100-fold lowering of the dissociation constant for Ca2+-binding to S100A1 when compared to binding in the absence of target [24,25], and makes this S100 interaction physiologically relevant within the cytoplasm [26].
INTRACELLULAR FUNCTIONS
S100A1
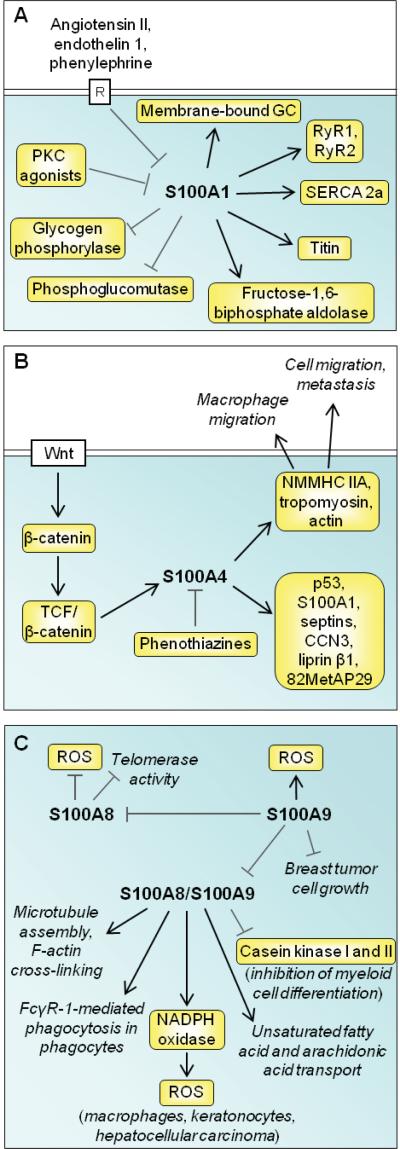
S100A1 is abundantly expressed in skeletal muscle fibers, cardiomyocytes and certain neuronal populations [3]. Within these cells, it is found diffusely in the cytoplasm and associated with cytoskeletal components and mitochondria. The S100A1 promoter contains several negative regulatory motifs controlled by inhibitory transcription factors downstream of G-protein-coupled receptors and protein kinase C (PKC) [27] (Fig. 3A). Accordingly, chronic stimulation of cardiomyocytes with angiotensin II, endothelin 1, phenylephrine and PKC agonists, which cause hypertrophic growth, reduces S100A1 mRNA and protein levels [28] (Fig. 3A). S100A1 interacts with the sarcoplasmic reticulum Ca2+-ATPase and RyR2 in the heart, resulting in improved Ca2+ handling and contractile performance [29] (Fig. 3A). It also targets the cardiac sarcomere and mitochondria, thereby reducing pre-contractile passive tension and enhancing oxidative energy generation [29] (Fig. 3A). S100A1 deficiency results in abnormal sarcoplasmic reticulum Ca2+ content and fluxes, accelerated deterioration of cardiac performance and transition to heart failure [29,30] and S100A1 gene delivery rescues failing myocardium [31]. In skeletal myofibers S100A1 binds to RyR1 and potentiates its open probability and plays role in skeletal muscle excitation-contraction coupling [24,32] (Fig. 3A). S100A1 also interacts with the giant sarcomeric kinase, titin, with potential improvement of sarcomeric compliance [30] (Fig. 3A). It also stimulates membrane-bound guanylate cyclase in photoreceptors likely involved in dark adaptation (reviewed in [3]) and regulates energy metabolism by stimulating fructose-1,6-biphosphate aldolase and inhibiting phosphoglucomutase and glycogen phosphorylase [3].
Fig. (3).
Schematic representation of proposed intracellular effects of S100A1, S100A4, and S100A8/S100A9. (A) S100A1 expression is negatively controlled by transcription factors downstream of G-protein-coupled receptors and PKC. S100A1 regulates energy metabolism and Ca2+ efflux from Ca2+ stores, stimulates striated muscle contraction, and activates a membrane-bound form of guanylate cyclase (GC) in photoreceptors in relation to dark adaptation. (B) S100A4 is induced by a Wnt/APC/GSK3/β-catenin/TCF pathway and targets several intracellular factors including NMMHC IIA, tropomyosin and actin with ensuing stimulation of cell migration and metastasis. Phenothiazines blocks intracellular S100A4 interactions. (C) S100A8 reduces telomerase activity and ROS production under the negative control of S100A9. S100A9 promotes ROS production, reduces breast cancer cell growth and negatively regulates S100A8/S100A9 heterotetramer complex activities as shown.
S100A2
S100A2 expression is downregulated in many cancers and loss in nuclear expression is associated with poor prognosis [33]. Thus, S100A2 is a tumor-suppressing protein binding to p53 transactivation domain and potentiating p53 is a potential mechanism [34]. However, S100A2 is upregulated in some cancers and other functions are unclear [33].
S100A3
S100A3 is highly expressed in hair root cells and some astrocytomas. It is proposed to have a role in epithelial cell differentiation and Ca2+-dependent hair cuticular barrier formation [35]. It may protect hair from oxidative damage due to very high Cys content [36].
S100A4
S100A4 expression is associated with outcome in patients witha number of tumor types by stimulating cell survival, motility, and invasion [37,38]. S100A4 interacts with cytoskeletal proteins such as nonmuscle myosin heavy chain (NMMHC) IIA, tropomyosin and actin, processes that can increase cell migration (Fig. 3B). A direct role of S100A4 in metastatic progression is proposed on the basis of its interaction with NMMHC IIA [39]; phenothiazines inhibit this interaction by inducing S100A4 oligomerization [40] (Fig. 3B). Deletion of s100a4 also results in defective macrophage migration and macrophage responses to chemotactic stimuli due to altered NMMHC IIA dynamics [41]. Several other binding partners for S100A4 have been identified in vitro, including the tumor suppressor p53, S100A1, the GTP-binding septins, the matricellular biomolecule CCN3, the leukocyte common antigen-related transmembrane tyrosine phosphatase-interacting protein liprin 1, and the tumor suppressor methionine aminopeptidase 82MetAP29 (Fig. 3B). However, the majority of these interactions have not been confirmed in vivo, and whether any of these binding proteins are involved in S100A4-induced metastasis is unknown. S100A4 is upregulated by heterotetrameric β-catenin/T-cell factor complex with resulting stimulation of tumor cell migration and invasiveness [42]. Accordingly, blockade of β-catenin downregulates S100A4 expression and reduces cell migration and invasion [43] (Fig. 3B). Intracellular S100A4 expression has also been associated with transcriptional regulation of matrix metalloproteinases (MMPs) and E-cadherin and it is not known whether this is attributed to cytoplasmic or nuclear S100A4 (or both) and mechanisms remain unidentified. Apart from the functions mentioned above, the biological role of nuclear S100A4 remains uncharacterized.
S100A5
S100A5 is upregulated in bladder cancers [44] and recurrent grade I meningiomas [45], but its biological function is unknown.
S100A6
S100A6 is implicated in cell proliferation, cytoskeletal dynamics and tumorigenesis [46,47]. It interacts with calcyclin-binding protein/Siah-1-interacting protein, a component of ubiquitin ligase involved in ubiquitination of β-catenin. S100A6 also inhibits interactions between the heat shock proteins (Hsp70 and Hsp90) and Sgt1 or Hop, suggesting a potential role in cell responses to different stressors. In this respect, the presence of S100A6 favors apoptosis in some cells [48], but limits it in others [49]. S100A6 also interacts with caldesmon, calponin, tropomyosin and kinesin light chain although functional manifestations are still unclear.
S100A7
S100A7 promotes aggressive features in breast cancer by binding to c-Jun activation domain-binding protein 1 thereby stimulating Akt and NF-κB [50]. Proinflammatory cytokines upregulate S100A7 expression in human breast cancer [51]. However, the tumorigenic activity of S100A7 appears to be restricted to estrogen receptor α-negative breast cancers, because in estrogen receptor α-positive breast cancers the protein appears to reduce the activation of the β-catenin/T cell factor 4 pathway with consequent reduction of uncontrolled proliferation [52].
S100A8
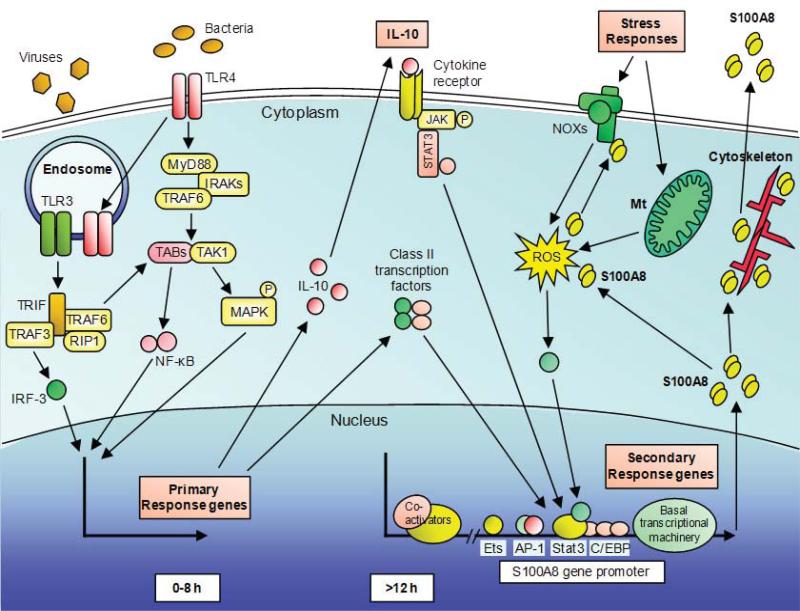
Gene deletion of S100A8 in mice is embryonic lethal, suggesting an important non-redundant function [53]. It comprises some 20% of the neutrophil cytoplasm. It is found in the nucleus of some cells [54]. S100A8 is induced in macrophages, dendritic cells, microvascular endothelial cells but not endothelial cells from larger vessels, epithelial cells (e.g. keratinocytes) and fibroblasts by pro-inflammatory stimuli [9]. In murine macrophages, S100A8, but not S100A9, is induced by TLR agonists in an interleukin (IL)-10-dependent manner; some agonists require cAMP and/or PGE2 generation for full expression. Our recent data indicates direct induction of S100A8 by oxidative stress in macrophages and this is amplified by the anti-inflammatory cytokine, IL-10. Mechanisms regulating S100A8 gene induction in macrophages are shown in Fig. (4). In murine keratinocytes, S100A8 is induced by oxidative stress whereas S100A9 is not, confirming discrete roles for these S100 proteins. In keratinocytes, S100A8 is entirely cell-associated and high nuclear expression is obvious [54].
Fig. (4).
Schematic representation of S100A8 induction in macrophages. LPS from bacteria is recognized by the surface receptor TLR4, activating MyD88-dependent and independent pathways. IRAKs and TRAF6 are recruited to MyD88 and subsequently activates a complex of TAK1 and TABs resulting phosphorylation of IκB and nuclear translocation of NF-κB. Simultaneously, TAK1 activates MAP kinase cascades leading to activation of AP-1. For the MyD88-independent pathway, TLR4 translocates to the endosome together with TRAM. In addition, TLR3 in the endosome, recognizes viral dsRNA. TLR3 and TLR4 activate TRIF-dependent signaling and subsequently activate NF-κB and IRF3. TLR-3 and TLR-4 activation triggers S100A8 gene induction in macrophages, but requires other factors. Induction is a late event that relies on de novo synthesized proteins, particularly IL-10, and class II transcription factors e.g. C/EBPs. AP-1 and Stat-3 bind to the S100A8 promoter. S100A8 is considered a stress response gene, and intracellular ROS generation either via NOXs or mitochondria (Mt) may be essential for induction. Intracellular S100A8, together with S100A9, can interact with components of the cytoskeleton and may mediate their rearrangements and dynamics. S100A8 and S100A9 directly bind to components of the NOX complex and mediate its activity. On the other hand, S100A8 is a potent oxidant scavenger and oxidative modifications of S100A8 can change its functions. S100A8 is actively secreted via a non-classical pathway which requires a functional microtubule network to exert its extracellular functions.
S100A8 is implicated in myeloid cell differentiation [55] and inhibits differentiation-dependent telomerase activity in a keratinocyte cell line in a Ca2+-dependent manner [56] (Fig. 3C). S100A8 scavenges intracellular reactive oxygen species (ROS) generated by activated neutrophils and may stabilize nitric oxide (NO) in these cells [57] (Fig. 3C). S100A8 reduces p38 mitogen-activated protein kinase (MAPK)-dependent phosphorylation of S100A9 in neutrophils in a Ca2+-dependent manner, thereby regulating their transendothelial migration [58]. Thus, S100A8 stimulates keratinocyte differentiation via inhibition of telomerase activity and exerts anti-inflammatory effects.
S100A9
S100A9 abrogates S100A8-induced reduction in telomerase activity [56] (Fig. 3C). S100A9 inhibits myeloid (dendritic cell and macrophage) differentiation and accumulation of myeloid-derived suppressor cells in pathological responses via intracellular ROS generation, thereby contributing to tumor growth [59] (Fig. 3C). S100A9 may differentially modify the phenotypic states of myeloid cells: S100A9-deficient neutrophils produce reduced amounts of cytokines in response to TLR-4 stimulation, S100A9-deficient dendritic cells produce more cytokines after TLR stimulation, and macrophages rapidly loose S100A9 expression during maturation. S100A9 gene deletion compromises neutrophil responses to particular chemoattractants and some aspects of skeletal dynamics may be compromised, resulting in impaired transendothelial cell migration [60]. S100A9 is a p38 MAPK target [61], phosphorylated after phagocyte activation. S100A9 reduces microtubule polymerization and F-actin cross-linking by the S100A8/S100A9 complex [58] and mediates Ca2+ signaling associated with inflammatory agonist-induced IP3-mediated Ca2+ release in neutrophils [62] (Fig. 3C). S-glutathionylated S100A9 is involved in glutathione metabolism in activated neutrophils [63]. STAT3 mediates S100A9 expression in some cancer cells and expression correlates with growth suppression [64]. In MCF-7 breast tumor cells S100A9 is essential for oncostatin M-induced growth repression [64]. In esophageal squamous cell carcinoma, S100A9 is a p53 transcriptional target and mediates the p53 apoptosis pathway [65]. S100A9 mediates transformation and proliferation of human aortic smooth muscle cells [66]. Thus, S100A9 exerts particular effects in a cell-specific manner.
S100A8/S100A9
S100A8 and S100A9 form a heterocomplex. S100A8/S100A9 inhibits casein kinase I and II [67] suggesting a role in myeloid cell differentiation [55,68] (Fig. 3C) and interacts with nuclear factors [69]. S100A8/S100A9 transports unsaturated fatty acids and arachidonic acid [70] and promotes NADPH oxidase activation in phagocytes by interaction with p67phox and Rac-2 [71]. S100A8/A9 is important in FcγR-1-mediated phagocytosis that requires depletion of intracellular Ca2+ stores for internalization; following phagocytosis of opsonized zymosan, S100A8/A9 acts as a cytoplasmic Ca2+ sensor that links Ca2+ influx to phagosomal ROS production [72]. S100A8 and S100A9 overexpression in HaCaT keratinocytes increases NADPH oxidase activity and enhances ROS levels (Fig. 3C); in hepatocellular carcinoma cells, co-expression of the two proteins promotes malignant progression by induction of ROS, down-regulation of p38 MAPK signaling, and cell survival and resistance to tumor necrosis factor (TNF)-α-induced apoptosis [73]. The cytoplasmic S100A8/S100A9 complex translocates to the membrane following phagocyte activation and may promote formation and stabilization of microtubules and enhance tubulin polymerization in neutrophils [58]. Interactions with cytoskeletal components are Ca2+-dependent and are important for migration, degranulation, phagocytosis of activated monocytes and neutrophils; the tetramer promotes microtubule polymerization and F-actin cross-linking [58,74] (Fig. 3C). S100A8/S100A9 expression is associated with a macrophage subtype associated with low antimycobacterial activity [75]; S100A8/S100A9-expressing epithelial cells resist invasion by P gingivalis, L. monocytogenes, and S. typhimurium [76]. S100A8/S100A9 co-expression in ductal carcinomas of breast is associated with poor tumor differentiation, vessel invasion, and node metastasis [77] (Fig. 3C): annexin 6 is involved in the Ca2+-dependent expression of S100A8/S100A9 on surface of tumor cells, and the annexin 6/S100A8/S100A9 complex may mediate cell membrane-regulated events [78]. S100A8/S100A9 may mediate pathological differentiation of psoriatic keratinocytes; it interacts with keratin intermediate filaments and may modulate wound healing [79].
S100A10
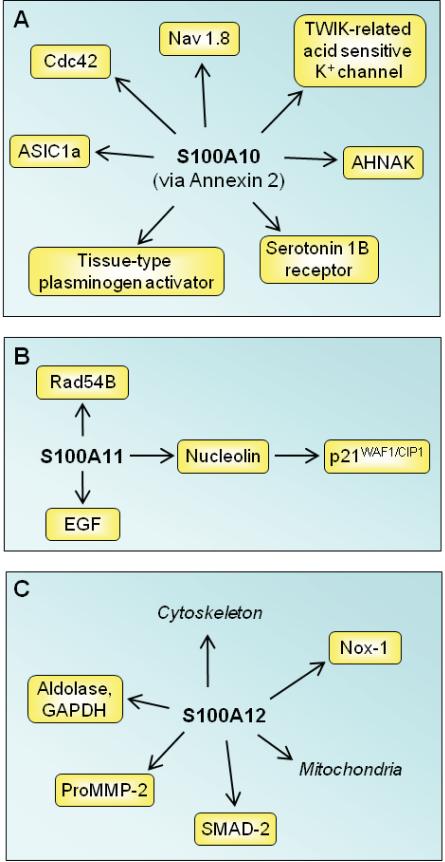
S100A10 tethers certain membrane proteins (i.e., the small GTPase of the Rho family Cdc42, tetrodotoxin-resistant sodium channel Nav 1.8, background two-pore domain potassium channel TWIK-related acid sensitive K, acid-sensing ion channel ASIC1a, actin-binding protein AHNAK, tissue-type plasminogen activator [tPa], serotonin 1B receptor) to annexin 2 thereby assisting their traffic to the plasma membrane and/or their firm anchorage at certain membrane sites [80,81] (Fig. 5A). A potential mechanism of S100A10/annexin 2/AHNAK ternary complex formation acting as a platform for membrane repair has been recently proposed [82]. S100A10 is downregulated in human and rodent depressive-like states and is implicated in the mechanism of action of antidepressant drugs and electroconvulsive seizures, in part due to its interaction with specific serotonin receptors [80,81] (Fig. 4A). S100A10 is induced by neurotrophins [83].
Fig. (5).
Schematic representation of proposed intracellular effects of S100A10, S100A11 and S100A12. (A) S100A10 is implicated in the mechanism of action of antidepressant drugs via interaction with serotonin 1B receptor. By binding annexin 2, S100A10 assists the traffic of several membrane proteins to plasma membranes. (B) S100A11 participates in the regulation of cell cycle by several mechanism as shown. (C) S100A12 regulates cytoskeleton-membrane interactions and has Ca2+-dependent chaperone/anti-chaperone-like functions.
S100A11
When phosphorylated by PKC-α, Ca2+-bound S100A11 inhibits cell growth via binding to nucleolin, translocation to the nucleus, and activation of the cell cycle modulator p21WAF1/CIP1 [84] (Fig. 5B). S100A11 also binds Rad54B, a DNA-dependent ATPase involved in recombinational repair of DNA damage [85], and stimulates cell growth by enhancing the level of epidermal growth factor (EGF) family proteins [86].
S100A12
S100A12 is constitutively expressed in neutrophils and inducible in macrophages and smooth muscle cells. S100A12 [87] may modulate interactions between cytoskeletal elements and membranes [88] (Fig. 5C). It inhibits aggregation of aldolase and GAPDH and may have Ca2+-dependent chaperone/anti-chaperone-like functions [89] (Fig. 5C). S100A12 expression in epithelial cells is associated with growth arrest [90]. S100A12 may play a role in vascular remodeling; it is expressed in human aortic aneurysms. Overexpression causes several vascular smooth muscle cell (VSCM) dysfunctions such as increased proMMP2 generation, increased phosphorylation and nuclear translocation of Smad2 and modulation of mitochondrial function [91] (Fig. 5C). VSCMs from mice overexpressing S100A12 have increased NADPH oxidase-mediated generation of peroxide, possibly via interaction with Nox-1 [92] (Fig. 5C). S100A12 potentiation of atherogenesis in Apo-E-/- mice, and increased vessel calcification via upregulation of multiple osteogenesis-related genes may occur through changes in ROS production [92]. On the other hand, S100A12 attenuated chemokine secretion in activated human airway SMC. TNF-α and interferon (IFN)-γ exposure enhanced CCL9, and CXCL10 mRNA and protein levels, and these were attenuated in S100A12 overexpressing cells, with the net effect of dampening allergic inflammation in the airway [93].
S100A13
S100A13 plays an important role in stress-induced release of fibroblast growth factor (FGF)-1 and IL-1α from several cell types including fibroblasts, osteoblasts and melanoma cells [94].
S100A14
S100A14 may function as a cancer suppressor affecting the p53 pathway [95] and modulating expression of matrix metalloproteinases, MMP1 and MMP9 [96]. However, recent studies show that S100A14 may play a dual role in tumor cells [97]; it acts as a negative regulator of p53 in cells expressing wild-type p53 likely decreasing p53 protein stability, which results in enhanced expression of MMP2 levels and consequent increase in tumor cell invasiveness; however, in tumor cells expressing mutant p53, S100A14 reduces MMP2 levels with consequent decrease in invasiveness.
S100A15
No intracellular role for S100A15 has been reported, the protein mainly acts as an extracellular factor [98].
S100A16
S100A16 is upregulated in several tumors [99]. It acts as a novel adipogenesis-promoting factor, having a negative impact on insulin sensitivity [100].
S100B
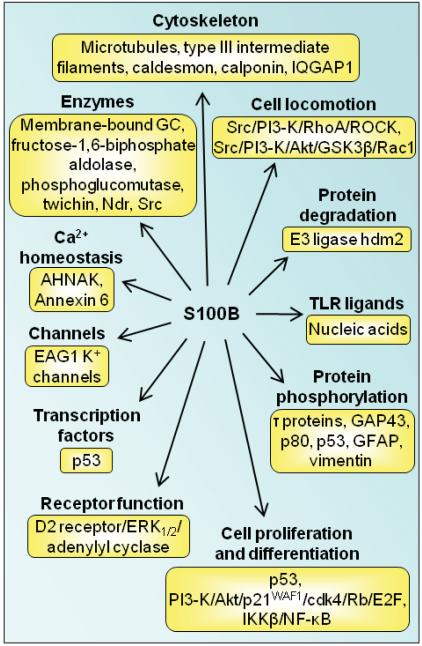
S100B is expressed in astrocytes, certain neuronal populations, Schwann cells, melanocytes, chondrocytes, adipocytes, skeletal myofibers and associated satellite cells, certain dendritic cell and lymphocyte populations and a few other cell types [7]. It acts as a stimulator of cell proliferation and migration and an inhibitor of apoptosis and differentiation [101-111] (Fig. 6), which might have important implications during brain, cartilage and skeletal muscle development and regeneration/repair, activation of astrocytes in the course of brain damage and neurodegenerative processes, and of cardiomyocyte remodeling after infarction, as well as in melanomagenesis and gliomagenesis. In particular, downregulation of S100B expression in precursor cells in a defined temporal window appears to be permissive for cell differentiation [101-106,108-110]. Sex-determining region Y-type high mobility group box 5, 6 and 9 (the so-called SOX trio), NF-κB, EGF and the Th-1-derived cytokine IFN-γ, regulate S100B expression in several cell types [see 7 for review; also see 12]. However, cells that downregulate S100B expression at the onset of their differentiation resume S100B expression at completion of development [7,106], and in mature cells the protein regulate a large variety of key activities including maintenance of shape, transcription, protein degradation, Ca2+ homeostasis, energy metabolism and enzyme functions by interacting with a wide array of target proteins. Binding partners of S100B within cells are tubulin and the microtubule-associated τ protein, the actin-binding protein caldesmon, calponin, type III intermediate filament subunits, annexin 6 [7,112-119], membrane-bound guanylate cyclase, the small GTPase Rac1 and Cdc42 effector IQGAP1, Src kinase, the serine/threonine protein kinase Ndr, the tumor suppressor p53, intermediates upstream of IKKβ/NF-κB, the giant phosphoprotein AHNAK/desmoyokin, the E3 ligase hdm2, dopamine D2 receptor and the mitochondrial AAA ATPase, ATAD3A [7,120,121] (Fig. 6). Thus, lack of S100B downregulation may maintain cell proliferation with potential beneficial effects during development and tissue regeneration, and detrimental effects during tumorigenesis. S100B also regulates Ca2+ homeostasis [122-125], but opposing results were reported in astrocytes and VSMCs [122,124] (Fig. 6). Moreover, S100B binds to, and inhibits EAG1 potassium channels Ca2+-dependently (Fig. 6) raising the possibility that its negative effects on cell differentiation may be via this mechanism as well [125]. Chronically high S100B levels such as those obtained in S100B transgenic mice are proposed to be causally correlated with Parkinson's disease likely via downregulation of dopamine D2 receptor and G protein-coupled receptor kinase2 expression, increased dopamine synthesis and metabolism, and decreased serotonin levels [126] and/or S100B interaction with the third cytoplasmic loop of the dopamine D2 receptor and extracellular signal-regulated kinase (ERK)1/2-mediated inhibition of adenylyl cyclase activity in striatal neurons [127] (Fig. 6). S100B is highly expressed in astrocytes [7] and to a lesser extent in certain neuronal populations [128,129], and its elevation in serum positively correlates with mood disorders [130] and schizophrenia [131]. Serum levels of S100B are of prognostic value in patients with cutaneous melanoma [105] and breast cancer [132]. Whether serum levels of S100B are an outcome predictor in severe traumatic brain injury is a matter of debate [133,134].
Fig. (6).
Schematic representation of proposed intracellular effects of S100B. S100B interacts with several intracellular proteins as shown thereby regulating protein phosphorylation, enzyme activities, the state of assembly of certain cytoskeleton components, the transcription factor p53, protein degradation, cell proliferation, locomotion and differentiation, dark adaptation of photoreceptors, Ca2+ homeostasis and the innate inflammatory response.
S100G
S100G is not a full member of the S100 sub-family and represents the only monomeric family member. Its major function is to act as cytosolic Ca2+ buffers in many tissues, resulting in modulation of Ca2+ adsorption [135]. A second designated function was on the interaction of S100G with phospholipids and may play a role for the transport of S100 proteins through membranes [136].
S100P
S100P interacts with ezrin/radixin/moesin thereby activating ezrin and promoting transendothelial migration of tumor cells [137]. S100P preferentially disperses NMMHC IIA fibers and subsequently reduces focal adhesion sites and cell adhesion thereby promoting cell migration and potentially metastasis [138]. S100P also interacts with the scaffolding protein IQGAP thereby reducing IQGAP ability to stimulate MAPK activity [139].
S100Z
S100Z is downregulated in several tumors [140], but no functional roles are reported.
SECRETION AND RELEASE OF S100 PROTEINS
Various S100 proteins are found in body fluids, including serum, urine, seminal plasma, saliva, sputum, cerebrospinal fluid and in feces and abscess fluid, principally associated with active disease states. Some, such as the S100A8/S100A9 complex and S100B are considered biomarkers for particular disease processes [74,105,141-147] (also see below). Secretion of some S100 proteins is stimulated by particular cell activators. For example, serotonin (5-HT1A) receptor agonists, antidepressants, glutamate, adenosine, IL-1β, lysophosphatidic acid and changes in extracellular Ca2+ and K+ levels trigger release of S100B from astrocytes [7,148-150], or of S100A4 from human pulmonary artery smooth muscle cells [5,151]. The selective serotonin reuptake inhibitor, fluoexitine, stimulates S100B secretion from serotoninergic neurons thereby downregulating micro-RNA16 in noradrenergic neurons, which consequently acquire properties of serotoninergic neurons [152]. Metabolic/oxidative stress induces release from several cells, and activation of some cell types or adhesion of some cells can also promote secretion. For example, TLR-2 activation on epithelial cells by Aspergillus conidia promotes release of S100B that binds the receptor for advanced glycation end-products (RAGE) on neutrophils in a paracrine manner, and mediates its association with TLR-2, causing TLR-2 inhibition [12]. In bronchial epithelial cells S100B is upregulated in the early stages of fungal infection via MyD88-dependent activation of canonical NF-κB and downregulated via TLR-3/9-dependent activation of non-canonical NF-κB at late stages [12]. Human CD8+ T cells and NK cells also express and secrete S100B following stimulation, thereby activating monocytes and neutrophils [153]. In addition to astrocytes [154], oligodendrocytes [155] and adipocytes [156] secrete S100B, and high S100B serum levels in schizophrenia are associated with insulin resistance [157].
S100 proteins lack a leader sequence and are not secreted via the classical Golgi pathway, and mechanisms remain somewhat unclear. S100A8/S100A9 may be passively released from necrotic myeloid cells, or actively secreted following translocation to the membrane, in a process requiring an intact microtubule network and PKC activation [58]. S100B also can be passively released from injured tissues [11,12]. S100 proteins may themselves have roles in nonclassical secretion. These proteins have diverse affinities to lipid structures that allow translocation across the plasma membrane following cell stress or activation. For example, S100A13-lipid interactions and formation of a multiprotein complex with FGF-1 (which also lacks a classical secretion sequence) and synaptotagmin peptides allows release, possibly mediated by N-type Ca2+ channel activity, or flip-flop to the extracellular compartment via annexin binding [158]. The heterotetrameric S100A10 and annexin 2 complex is associated with von Willebrand factor secretion from endothelial cells [159]. S100B associates with natural [160] and artificial [161] membranes; this, although not formally proven, might represent a mechanism of its secretion. In this respect, the interaction of S100G with phospholipid micelles has been proposed as a prototype of S100 protein interaction with membranes for subsequent passage through them [136]. Other mechanisms include vesicular S100A8/S100A9 release in neutrophil extracellular traps (NETs), triggered by production of ROS from dying neutrophils. NETs are chromatin fibers (histones and DNA) bound with antimicrobial proteins that deliver high local concentrations to pathogens [162].
S100 RECEPTORS
The oligomeric forms of some S100 proteins, and their putative binding partners can determine function [163]. For example, S100A8 and S100A9 have functions that can be dependent or independent of the heterocomplex. In some circumstances, more highly oligomerized S100 proteins may more functionally efficient and in some cases high concentrations of S100 proteins are required for activation whereas other functions depend on very low amounts [5], indicating different receptor affinities, different extents of ligand-induced receptor oligomerization or requirements of coreceptors. Divalent cation binding can also determine functional outcomes. In addition, some S100 proteins are structurally altered and particular posttranslational modifications can promote changes in function. These include oxidation products of S100A8 and/or S100A9 generated by NO, oxygen-free radicals and hypohalous acids [164,165], and of S100B [166].
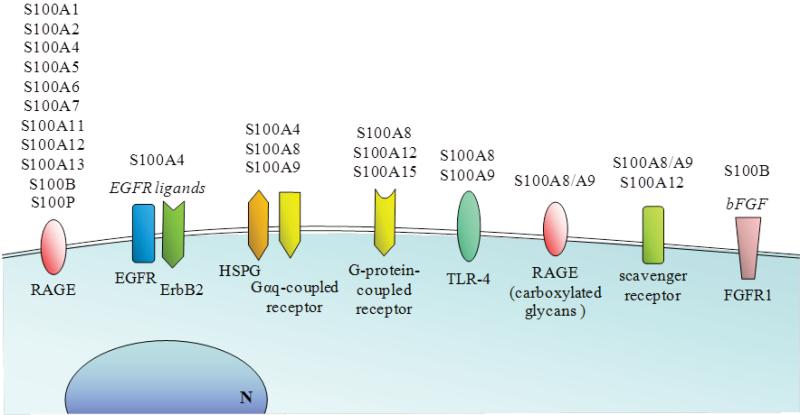
Receptors mediating extracellular functions of S100 proteins have been elusive and remain a matter of debate and there is evidence for multiple receptors. Both non-receptor- and receptor-mediated endocytosis is implicated. For example, exogenous S100A1 is internalized into neurons via multiple endocytic pathways and delivered to early/recycling endosomes, Golgi apparatus, late endosomes, and lysosomes [167]. Although many effects may be mediated by RAGE, others are not (Fig. 7). Structural studies of some S100 proteins indicate at least three recognition sites within two distinct surfaces that may accommodate multiple binding partners [168] that result in complex interactions, or binding to specific ligands on different target cells. This is supported by reports that some S100 functions reside within the divergent hinge domains between the Ca2+-binding regions and are mimicked by relevant peptides [169] whereas others require homo- or hetero-S100 complexes. Structural and binding data suggest that tetrameric/octameric S100B triggers RAGE by receptor dimerization [170]. Furthermore, some S100s, such as S100A6 preferentially bind the C2 domain of RAGE rather than the V domain that binds S100B and S100A12, indicating another layer of receptor complexity [171]. S100A12 binds RAGE in vitro with very low affinity but this increases >1000 fold when S100A12 is in the Ca2+-[172] or Zn2+-bound hexameric states [173]. Although S100A8/S100A9 are proposed to bind RAGE, particularly on tumor cells [174,175] (Fig. 7), only S100A9 in the presence of Ca2+ and Zn2+ has a high affinity; S100A8 has virtually none, and binding of S100A8/A9 is relatively weak [176]. Also, RAGE ligation by S100B results in RAGE/TLR-2 association in neutrophils leading to inhibition of TLR-2-provoked responses [12], and ligation of S100B to bFGF-bFGF receptor (FGFR1) complex on myoblasts (Fig. 7) results in recruitment of RAGE in a RAGE/ S100B/bFGF/FGFR1 tetracomplex that enhances bFGF/FGFR1 signaling and inactivates RAGE signaling [11]. Some outcomes of S100 protein-stimulated RAGE signaling are summarized in [5,177,178].
Fig. (7).
Schematic representation of the receptors involved in the transduction of S100 protein signaling. RAGE is an established receptor for several S100 protein members in a variety of cell types. By interacting with EGFR ligands and bFGF, S100A4 and S100B can also activate EGFR and FGFR1, respectively. By interacting with heparan sulfate proteoglycans (HSPG) S100A4, S100A8 and S100A9 also activate Gαq receptors. S100A8, S100A12 and S100A15 can activate G-protein-coupled receptors. S100A8 and/or and S100A9 interact with TLR-4 in phagocytes, and as a heterocomplex S100A8/S100A9 can bind to carboxymethylated RAGE. S100A8/S100A9 and S100A12 also activate scavenger receptors.
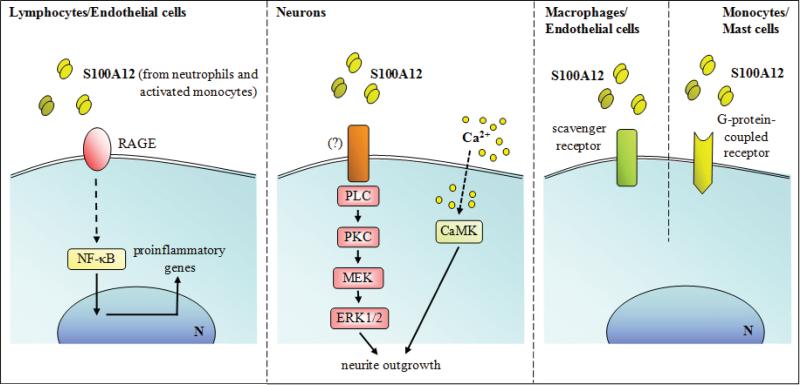
S100A12 was the first S100 protein for which RAGE was the designated receptor on myeloid cells [179] although RAGE participation is controversial [164] and other receptors including N-glycans (including glycosylated RAGE) [180], a G-protein-coupled receptor [169] and scavenger receptors [181,182] are implicated (Fig. 7). Glycosylated RAGE can form higher-order multimeric complexes with S100A12 which are reduced by deglycosylation or by non-glycosylated soluble RAGE. Thus carboxylated N-glycans on RAGE may enhance binding potential and promote receptor clustering upon oligomeric S100A12 binding [178]. In addition to RAGE, receptors for S100A8 and or S100A9 include the scavenger receptor CD36 [183,184], and heparan sulfate proteoglycans and carboxylated N-glycans [184] (Fig. 7). In one study, TLR-4 is proposed as an S100A8 receptor and S100A9 inhibits binding [60] (Fig. 7). The S100A8/A9 complex does not activate TLR-4 but enhances cytokine production by bone marrow cells stimulated with lipopolysaccharide (LPS) [60]. In contrast, another study comparing S100A8 and S100A9 binding to TLR-4/MD2 showed high affinity S100A9 binding that was enhanced by Ca2+ and Zn2+; binding of the S100A8/S100A9 complex was 5-fold less. Moreover, binding sites in S100A9 for TLR-4/MD2 appear to be the same as those for RAGE [176]. Other examples include effects of S100A4 on neurite outgrowth that depend on binding to heparan sulphate proteoglycans and a putative Gαq-coupled receptor [185].
EXTRACELLULAR FUNCTIONS OF S100 PROTEINS
S100A1
S100A1 is found in the extracellular compartment after heart ischemia. It enhances Ca2+ influx in cultured ventricular cardiomyocytes [186], and Cav1 channel currents in a protein kinase A-dependent manner, prolonged action potentials, and amplified action potential-induced Ca2+ transients in neurons [167].
S100A2
S100A2 is chemotactic for eosinophils [187]. S100A2 may be involved in calcification of cartilage/bone [188].
S100A3
No extracellular function has been reported.
S100A4
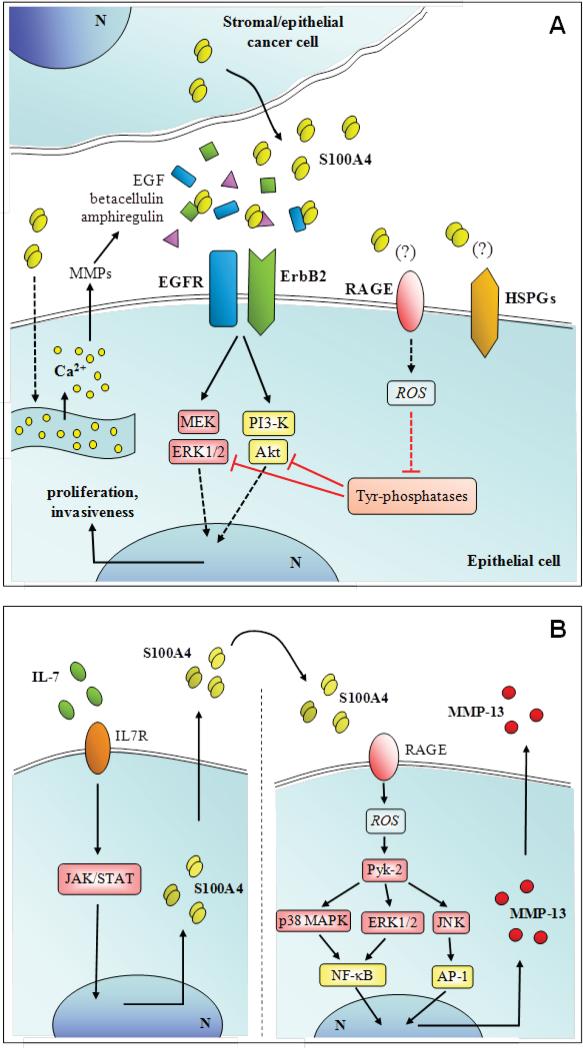
S100A4 has effects on numerous cell types. S100A4 released by tumor or stroma cells triggers pro-metastatic cascades by modifying the cytoskeleton and focal adhesions of tumor cells, downregulating the pro-apoptotic Bax and the angiogenesis inhibitor thrombospondin-1 genes, and increasing production of MMPs by endothelial and tumor cells [189]. S100A4 induces tube formation in endothelial cells in a RAGE-independent pathway, possibly through interactions with annexin 2 and accelerated plasmin formation [190]. S100A4 has various effects on leukocyte migration. It stimulates cytokine production, particularly granulocyte colony-stimulating factor and eotaxin-2 from T lymphocytes [191], and may thereby influence allergic inflammation as it is expressed in normal myeloid cells [192]. It may also stimulate T cell infiltration into primary tumors [191]. S100A4 is released under the action of the chemokine RANTES (CCL5) via microparticle shedding from the plasma membrane of tumor and stromal cells, and in turn extracellular S100A4 cooperates with RANTES (CCL5) in promoting tumor progression [193]. Extracellular S100A4 may also regulate tumor progression by interacting with EGF receptor (EGFR) ligands, thereby enhancing EGFR/ErbB2 receptor signaling and cell proliferation [194] (Fig. 8A). S100A4 may promote TCRγΔ T-cell mediated lysis [195] and negatively regulate matrix mineralization/calcification [196]. S100A4, induced in articular chondrocytes by IL-7, stimulates MMP-13 production and release in a RAGE-dependent manner [197,198] (Fig. 8B). S100A4 may be cardioprotective by promoting smooth muscle cell motility and proliferation, and possibly mediating neointimal hyperplasia and arterial muscularization, and promoting cardiac myocyte growth, survival, and differentiation [199]. Oligomeric S100A4 forms (tetrameric or more) promote growth and survival of cultured neurons, possibly protective after injury and regulates astrocyte motility. ERK1/2 is activated in many of the S100A4-responsive cells and may modulate growth and survival responses of S100A4 [200].
Fig. (8).
Schematic representation of proposed extracellular effects of S100A4 on epithelial tumor cells (A) and articular chondrocytes (B).
S100A5
No extracellular function has been reported.
S100A6
S100A6 is expressed by many cells and tumors [46,47]. It modulates RAGE-dependent survival of neuroblastoma cells by triggering apoptosis and generation of ROS through c-Jun NH2 terminal protein kinase activation [171]. S100A6 may regulate secretory processes in some cells. It stimulates secretion of lactogen II by trophoblasts and insulin release from pancreatic islet cells. S100A6 may modulate allergic responses by inhibiting histamine release by mast cells [46,47].
S100A7
S100A7 (psoriasin) is overexpressed in inflammatory skin diseases and induced in keratinocytes by IL-17 and IL-22 [201] and by the TRL-5-ligand, flagellin [202]. It has roles in antimicrobial responses and innate immunity. S100A7 adheres to, and reduces E. coli survival; the hinge region (amino acids 35-80) is sufficient for full activity [203,204]. S100A7/RAGE and Zn2+ binding are required for chemotactic activity for lymphocytes, monocytes and granulocytes, and S100A7 acts synergistically with S100A15 [205]. Mice expressing elevated amounts of doxycycline-regulated mS100A7/A15 in skin keratinocytes have an exaggerated inflammatory response characterized by leukocyte infiltration and elevated levels of T helper 1 and T helper 17 proinflammatory cytokines, linked to the pathogenesis of psoriasis [205, 206]. S100A7 promotes α-secretase activity by promoting ADAM (a disintegrin and metalloproteinase)-10 production from primary cortico-hippocampal neuron cultures and in brain. In so doing, it may prevent generation of amyloidogenic peptides in Alzheimer's disease [207]. S100A7 stimulates ROS generation from neutrophils [206].
S100A8
S100A8 is implicated in regulation of inflammation. Deletion of the gene in mice indicates a non-redundant function that may involve an immunoregulatory role in maternal-fetal tolerance [53]. Murine S100A8 and its hinge domain are chemotactic for leukocytes at picomolar concentrations. These promote actin polymerization in monocytes and neutrophils, likely via a G-protein-coupled receptor [208-210]. Human S100A8 is chemotactic for neutrophils and activity may depend on its oxidation state [87]. Murine S100A8 provokes a mild and transient influx of neutrophils and monocytes when injected intradermally or intraperitoneally (i.p.), with kinetics similar to those elicited by a delayed-type hypersensitivity reaction [211]. The chemotactic properties of murine S100A8 are modified by oxidation, particularly by hypohalous acids generated from activated phagocytes [164,165] and these effects are obvious in vitro and following injection in vivo. S100A8 is also chemotactic for periodontal ligament cells [212]. Effects of S100A8 on leukocyte adhesion are controversial. It was reported to stimulate neutrophil adhesion to fibrinogen [209] whereas others found S100A8 suppressed expression of the high affinity β-2 integrin epitope on neutrophils induced by S100A9, thereby likely reducing leukocyte adhesion [213]. S100A8 is reported to induce TNF-α and IL-1β production from murine bone marrow cells via TLR-4 activation, and induction was negated by S100A9 [214]. However this may be a property only exhibited by neutrophils activated with S100A8 [60]. S100A8 activates FcγRI and FcγRIV on macrophages through the activation of TLR-4 [72,214], and upregulates and activates MMPs and aggrecanase enzymes from chondrocytes suggesting a role in pericellular matrix degradation [215]. However S100A8 can also inhibit MMP activity, suggesting an auto-regulatory mechanism [216].
The S100A8 gene is upregulated in several cell types by the anti-inflammatory agent corticosteroid, and in macrophages its induction by TLR agonists depends on the anti-inflammatory cytokine IL-10 [9,217,218]. S100A8 induction in some cells may be dependent on ROS generation. This is implicated in its induction in endotoxin-activated macrophages (K. Hsu and C.L. Geczy, in preparation) and is involved in S100A8 expression in keratinocytes provoked by UV irradiation [54]. Some of the factors regulating S100A8 gene induction in macrophages are depicted in (Fig. 4).
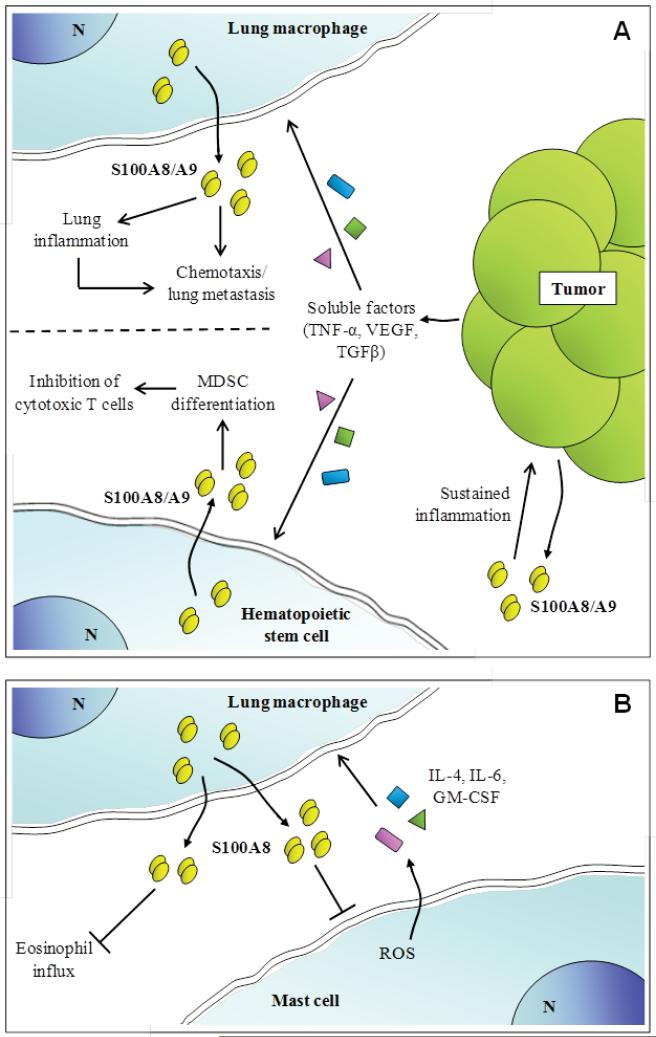
S100A8 has anti-inflammatory effects by triggering oxidation-sensitive repulsion of neutrophils [219] and is an effective 2-electron oxidant scavenger and at high concentrations, may protect from oxidative damage in acute and chronic inflammatory lesions [220]. S100A8 is protective in an acute asthma model as it suppresses mast cell activation by allergen by reducing intracellular ROS required for signaling, thereby suppressing production of key mediators required for eosinophil migration and reducing symptoms of allergic inflammation [221] (Fig. 9A). S100A8 is also readily S-nitrosylated by physiological NO donors and endogenous levels found in neutrophils suggest that it may regulate NO availability as it is a relatively stable adduct. S-nitrosylated S100A8 reduces mast cell activation and mast cell-mediated leukocyte adhesion and transmigration in the microcirculation in vivo, and shuttles NO to hemoglobin [57] (Fig. 9A). Thus S100A8-SNO formed during an inflammatory episode may contribute to maintenance of vessel patency in the microcirculation. Moreover, overexpression of S100A8 accelerates healing in wound models [222] and an Ala-Cys42 S100A8 mutant promotes more effective wound healing than the native form [223]. Thus the oxidantscavenging capacity of S100A8, and the functional modifications generated, may have important roles in resolution of inflammation (Fig. 9A).
Fig. (9).
Schematic representation of proposed extracellular effects of S100A8/S100A9. (A) Activated by TNF-α, TGF-β and VEGF secreted by distant tumors, lung macrophages release S100A8/S100A9 that promotes local inflammation and attract metastatic cells thus promoting tumor cell homing in the lung. Moreover, S100A8/S100A9 might sustain inflammation in the original tumor site. (B) It is also suggested that S100A8/S100A9 released by hematopoietic stem cells under the action of TNF-α, TGF-β and VEGF might inhibit cytotoxic T cells thereby contributing to tumor growth by several mechanisms.
S100A9
S100A9 affects leukocyte migration, adhesion and transmigration from blood vessels [209,224]. Murine S100A9 is chemotactic for alveolar and peritoneal macrophages [8] and human S100A9 is chemotactic for neutrophils [209]; it activates expression of high affinity β-2 integrin epitope on neutrophils [213] and promotes their adhesion to fibronectin, a property that may maintain these cells within the extravascular compartment [225]. S100A9 also promotes degranulation of secretory and specific/gelatinase granules from neutrophils [226] and enhances human neutrophil bactericidal activity [227]. One report indicates that S100A9 induces TNF-α, IL-1β, IL-6 and IL-8 in macrophages via NF-κB activation [228] whereas another claims that it principally activates neutrophils via TLR-4 [60] and S100A9 decreases phorbol-12-myristate-13-acetate-triggered peroxide production by BCG-activated macrophages [229]. In the presence of Zn2+ and Ca2+ S100A9 is a RAGE ligand and a TLR-4 ligand [177] and may contribute to the pathogenesis of autoimmune diseases. S100A9 is mitogenic for fibroblasts [230] and stimulates IL-8 release from epithelial cells [231]. S100A9 may mediate dystrophic calcification [220] and is incorporated into urinary calcium oxalate crystals [232].
S100A9 also has anti-inflammatory properties. S100A9 has neutrophil repelling activity that may suppress migration [233]. It also suppresses macrophage activation following uptake of apoptotic neutrophils by inhibiting NO, H2O2 and TNF-α production in vitro [234,235]. S100A9 is readily glutathionylated and this alters its pro-adhesive properties [63]. S100A9 can scavenge ROS, likely through methionine sulfoxide formation on several Met residues; oxidation or mutation of key Met residues negates fugetactic activity for neutrophils [233]. S100A9 may be protective in asthma. In a rat model, it decreased pulmonary resistance and increased compliance upon antigen challenge and significantly decreased the isometric tension of isolated tracheal spirals [236]. S100A9 also inhibits MMP activity by chelating Zn2+ from the active site of the enzymes [216].
Importantly, S100A9 may also regulate the magnitude of the acquired immune response by modulating levels of the co-stimulatory molecule, B7, expressed on antigen-presenting cells. Costimulation through the B7/CD28 pathway is important for activation of lymphocytes by alloantigens. S100A9 may modulate tolerogenic dendritic cell-T cell activation by reducing B7 levels and subsequent T-cell priming [237], and its over-expression inhibits dendritic cell differentiation [59].
A C-terminal peptide reduces spreading and phagocytosis of adherent macrophages induced by proteinase-activated receptor-1 agonists [234] and this peptide, like S100A9, suppresses macrophage activation following ingestion of apoptotic neutrophils [235]. S100A9 C-terminal peptide also modulates primary afferent nociceptive signals by inhibiting activation of N-type voltage operated Ca2+ channels and may reduce pain responses in inflammation [238].
S100A8/S100A9
The S100A8/S100A9 complex is also known as calprotectin. Heterodimerization with S100A8 stabilizes S100A9, causing elongation of its C-terminal α-helix, and tetramers form and expose two high affinity Zn2+-binding sites at the S100A8/S100A9 subunit interface, which may be important functional sites for functions of calprotectin [239,240]. Anti-microbial properties, particularly to fungi and Staphylococcus aureus, are chiefly mediated by chelation of Zn2+ and Mn2+ [9,240,241], and when produced by IL-1β-activated keratinocytes, S100A8/S100A9 contributes to the anti-invasive properties of skin [9]. However S100A8/S100A9 increases Mycobacterium tuberculosis growth in vitro, suggesting a role in immunopathogenesis of tuberculosis [242]. It also promotes HIV-1 transcriptional activity and viral replication in infected CD4+ T-lymphocytes [243], and upregulation of these S100 genes in monocytes by dsRNA may potentiate this effect [217]. In the aging prostate, amyloid deposition of S100A8/S100A9 associated with bacterial infection and macrophage activation may contribute to processes that increase risk of malignancy [244].
Human S100A8/S100A9 is chemotactic for neutrophils [209] and influences migration of other cell types, including myeloid-derived suppressor cells [245] and some tumor cells [174], and may facilitate tumor cell invasion [246,247] (Fig. 9B). S100A8/S100A9 may activate proinflammatory cytokine production by human monocytes and macrophages via the NF-κB and p38 MAPK pathways [228] and promotes tumor development via RAGE-mediated production of inflammatory mediators [248] (Fig. 9A). Furthermore, low concentrations of S100A8/S100A9 promote growth of some tumor cells through RAGE signaling and NF-κB activation [249], and binding to carboxylated glycans on RAGE promotes proliferation of colon cancer cells by this pathway [250]. Binding of the complex to RAGE on prostate cancer cells activates the MAPK pathway [174]. S100A8/S100A9-activated melanoma cells over-express MMPs -2, -9 and -14 [245]. On the other hand, S100A8/S100A9 inhibits MMPs by sequestering Zn2+ from their active sites [216].
S100A8/S100A9 potentiates the TLR-4 response of bone marrow cells to LPS, but does not directly activate TLR-4 [214]; it may induce NO production by macrophages [251]. On the other hand, S100A8/S100A9 may suppress acute inflammation by binding and modulating activities of pro-inflammatory cytokines [252]. S100A8 and S100A9 inhibit the spontaneous and stimulated oxidative burst of neutrophils, possibly mediated by P1 adenosine receptors [253]. The complex may also regulate lymphocyte functions. It stimulates CD8+ T lymphocytes from individuals with lupus erythematosus to produce IL-17, suggesting a role in development of autoreactive lymphocytes [254] although it inhibits immunoglobulin synthesis [255]. S100A8/S100A9 is elevated in psoriatic lesions; the complex induces a number of cytokines and chemokines in normal human keratinocytes, and at low concentrations stimulates keratinocyte growth [256]. S100A8/S100A9 may stimulate pro-inflammatory properties of endothelial cells, possibly mediated by RAGE and potentiates their activation by advanced glycation end-products [257]. S100A8/S100A9 delivers arachidonic acid to endothelium via CD36-mediated uptake [183] and stabilizes and protects leukotriene A(4) from nonenzymatic hydrolysis, possibly increasing availability of bioactive leukotrienes [258]. It also causes loss of contacts in microvascular endothelial cells in vitro, triggering apoptosis via caspase-dependent and independent mechanisms and is suggested to promote endothelial cell damage in vasculitis and inflammatory disease [259]. It also dissociates pancreatic acinar cell-cell contacts in a Ca2+-dependent manner [224].
Extracellular S100A8/A9 can affect cell growth and have cytotoxic/apoptotic effects. It inhibits proliferation and differentiation of C2C12 myoblasts and induces caspase-3-dependent apoptosis [260]. At relatively high concentrations, it inhibits growth of a variety of normal cell types (macrophages, bone marrow cells, lymphocytes, fibroblasts) (Fig. 9A), and has apoptosis-inducing activity to numerous tumor cell lines [261]. In some cells S100A8/S100A9 can reduce mitochondrial membrane potential, causing Smac/Diablo and Omi/HtrA2 (without cytochrome C) release and inhibition of mitochondrial fission machinery, Drp 1, inducing cell death by altering the balance between pro- and anti-apoptotic proteins [262]. S100A8/S100A9 may also promote autophagy-like death; apoptosis and autophagy may involve translocation of BNIP3, a BH3 only pro-apoptotic Bcl2 family member, to mitochondria, and cross-talk between mitochondria and lysozomes and generation of ROS [263]. Zn2+ chelation may be another mechanism [9,216,264]. However, s100a9-/- mice, which also lack S100A8 [265], show reduced formation of spontaneous prostate cancer and in EL-4 lymphoma model tumor growth inhibition was observed in s100a9-/- mice [266]; S100A9 is proposed to act as a tumor promoting factor via interaction with TLR-4.
A role for S100A8/A9 in the pathophysiology of atherosclerosis is proposed. The proteins are abundant in foam cells in atherosclerotic lesions [220] and because urokinase plasminogen activator upregulates S100A8/A9 expression in, and secretion from macrophages [267], the S100A8/A9 released may promote endothelial apoptosis. This could facilitate entry of monocytes and lipids into the artery wall [257]. S100A8 and S100A9 influence cardiomyocyte contractility by causing RAGE-dependent decreases in Ca2+ flux [268] and S100A9 in calcifying microvesicles from plaque may promote dystrophic calcification [232]. However studies in Apo-E low-density lipoprotein (LDL)-receptor-deficient, S100A9-deficient bone marrow chimeras question the role of S100A9 in atherogenesis [68].
S100A10
Heterotetrameric complexes of S100A10 with annexin 2 serve as extracellular binding partners for pathogens and host proteins. The promyelocytic leukemia-retinoic acid receptor α oncoprotein increases the expression of cell surface S100A10 which binds tPA and plasminogen via C-terminal lysine residues, promoting tPA-dependent plasmin production that can contribute to fibrinolysis as seen in the clinical hemorrhagic phenotype of acute promyelocytic leukemia [269]. Plasmin binds S100A10 at a distinct site; the S100A10-plasmin complex stimulates plasmin autoproteolysis thereby providing a highly localized transient pulse of plasmin activity at the cell surface [270]. Besides regulating fibrinolysis, S100A10 also plays an important role in angiogenesis in vivo, pointing to a critical role in endothelial cell function [271]. S100A10 mediates macrophage recruitment in response to inflammatory stimuli by activating pro-MMP-9 that in turn, promotes plasmin-dependent invasion in vivo [272]. As to its function as plasminogen receptor, S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites in vivo [273]. The tumor promoting properties of S100A10 might be reduced by the Rho GTPase-activating protein, DLC1, that competes with S100A10 for annexin 2 decreasing the steady-state level of S100A10 expression in a dose-dependent manner by displacing it from annexin 2 and making it accessible to ubiquitin-dependent degradation [274]. Interaction of S100A10 and a viral NS protein is essential for intracellular trafficking of nonenveloped bluetongue virus [275].
S100A11
S100A11 localizes in the cytosol of luteal cells in the mouse ovary, and oviductal epithelial cells, and suppresses fertilization through its action on cumulus cells [276]. S100A11 is induced/released by chondrocytes cultured with IL-1β, TNF-α, and CXCL8 [277]. It promotes hypertrophic chondrocyte differentiation, and stimulates RAGE-dependent type X collagen and IL-8 production by reticular chondrocytes. Transamidation generates covalently-bonded S100A11 homodimers that can signal through RAGE and activate the p38 MAPK pathway to accelerate chondrocyte hypertrophy and matrix catabolism that may promote osteoarthritis progression [278].
S100A12
There is no S10012 in rodent genomes [279,280]. It is constitutively expressed in neutrophils; TNF-α, IL-6 and endotoxin induce the gene in monocytes/macrophages, LPS in smooth muscle cells [281]. S100A12 inhibits growth and motility of filarial parasites by binding paramyosin [282]. Low amounts can immobilize microfilariae and high concentrations kill them, possibly by interruption of helminthic contractile elements. The C-terminal peptide (calcitermin) is antimicrobial and antifungal; unlike calprotectin, Zn2+ enhances activity [283].
Low concentrations of S100A12 and its hinge domain are chemotactic for monocytes and mast cells; a G-protein-coupled receptor is implicated [169] (Fig. 10). Higher levels activate mast cells and potentiate IgE-mediated activation in a RAGE-independent manner. S100A12 induced pro-inflammatory cytokine production from mast cells, particularly IL-6 and IL-8, chemokines important for neutrophil, monocyte and lymphocyte recruitment, and caused TNF-α release from pre-formed stores [284] (Fig. 10). On the other hand, overexpression of S100A12 in smooth muscle cells in lung resulted in S100A12 release with less production of some chemokines and reduced symptoms of acute asthma upon antigen challenge [93].
Fig. (10).
Schematic representation of proposed extracellular effects of S100A12 on lymphocytes, endothelial cells, neurons, and macrophages.
Bovine S100A12 stimulates RAGE-dependent TNF-α and IL-1β production from murine BV-2 microglial cells, IL-2 from lymphocytes and intercellular adhesion molecule-1 and vascular adhesion molecule expression on endothelial cells [179] (Fig. 10). However S100A12 does not induce cytokine production by human monocytes or macrophages [285]. S100A12 enhances Mac-1 integrin affinity and L-selectin shedding from neutrophils and modulates neutrophil release from bone marrow [286]. S100A12 stimulates neurite outgrowth of rat hippocampal neurons via RAGE ligation and activation of phospholipase C, PKC, CAM-kinase II and MAPK pathways [287] (Fig. 10). S100A12 binds to RAGE in the form of a hexamer [172]. S100A12 strongly inhibits MMP-3 and -9 by chelating Zn2+ from their active sites and complexes containing Zn2+ colocalize with these MMPs in atherosclerotic lesions, supporting this role in vivo [285]. Its overexpression in smooth muscle cells in mice leads to aortic aneurysms, linked to leukocyte influx, increased IL-6 in response to LPS, and increases of latent MMP-2 levels [91]. Overexpression of S100A12 in vascular smooth muscle cells in mice promotes atherosclerotic plaque remodeling and nodular calcification, possibly by influencing osteoblastic genes in a feedback mechanism involving RAGE [92]. Cu2+ sequestration by S100A12 may modulate redox [288].
S100A13
S100A13 promotes it own intracellular translocation, possibly via RAGE binding on endothelial cells [289]. S100A13 is involved in the non-classical secretion of FGF-1 [94,158]; the complex may contribute to angiogenesis [290].
S100A14
S100A14 stimulates proliferation and apoptosis in an esophageal squamous cell carcinoma cell line via RAGE engagement at low and high doses, respectively [291].
S100A15
S100A15 is expressed in keratinocytes in inflamed skin and can be induced by LPS, IL-1β and Th-1 cytokines [292]. It is chemotactic for monocytes and granulocytes, possibly via a G-protein-coupled receptor and acts synergistically with S100A7 in leukocyte recruitment in vitro and in vivo [201]. Human S100A15 has antimicrobial activity against E. coli [293].
S100A16
No extracellular function has been reported.
S100B
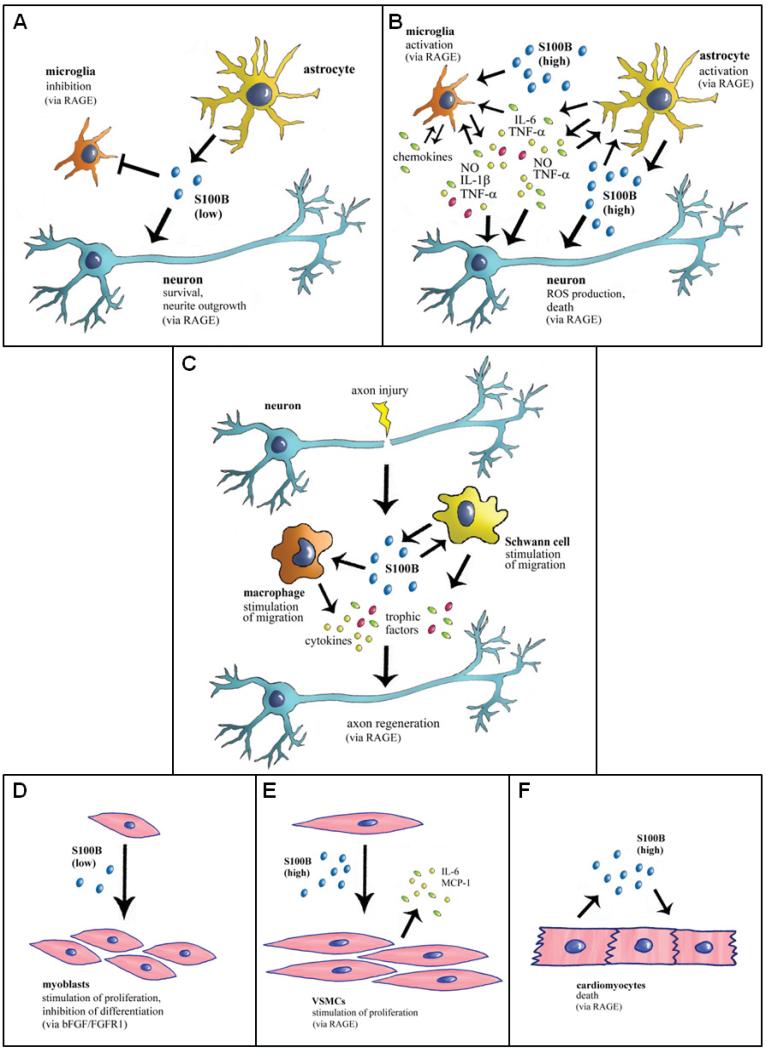
S100B secreted or released from astrocytes has different (trophic and toxic) effects on neurons, astrocytes and microglia depending on the concentration [7,294,295]. Up to a few nanomolar amounts S100B behaves like a neurotrophin protecting neuronal cells against neurotoxic stimuli through stimulation of ERK1/2 and NF-κB-mediated upregulation of the anti-apoptotic Bcl-2, whereas at micromolar doses it kills neurons through excess ERK1/2 stimulation and ROS production and/or potentiation of neurotoxic effects of β-amyloid, via RAGE engagement in both cases [7,294-297] (Fig. 11A, B). Others have shown that at high doses S100B still is pro-survival towards neurons via RAGE-dependent activation of phosphatidylinositol 3-kinase/Akt and NF-κB [171]. S100B stimulates astrocyte proliferation at low doses and promotes inflammatory activities in astrocytes at high doses [7,294-297] (Fig. 11A, B). Whereas at low doses S100B attenuates microglia activation via the STAT3 pathway [298,299], at high doses it activates microglia as evidenced by NF-κB- and AP-1-dependent the stimulation of cytokine expression and release and cyclo-oxygenase-2 expression [7,294,295,300-304] and stimulates microglia migration via NF-κB- and AP-1-dependent upregulation of chemokine expression and chemokine release and upregulation of chemokine receptors [305], in a RAGE-dependent manner (Fig. 11A, B). After permanent middle cerebral artery occlusion in S100B transgenic mice infarct volumes are significantly increased during the first days post-infarct and astrogliosis is enhanced compared with controls [306]. Moreover, S100B transgenic mice show increased susceptibility to perinatal hypoxia-ischemia [307], and overexpression of S100B accelerates Alzheimer disease-like pathology with enhanced astrogliosis and microgliosis [308]. Collectively, these results support the possibility that following accumulation in the extracellular space S100B might contribute significantly to neuroinflammation. However, intraventricular S100B infusion induces neurogenesis within the hippocampus, which has been associated with an enhanced cognitive function following experimental traumatic brain injury [309,310], and S100B is released by in vitro trauma and reduces delayed neuronal injury [311,312]. S100B is upregulated in experimental autoimmune encephalomyelitis, a model of multiple sclerosis, and activates RAGE in CD4+ T cells that infiltrate the central nervous system, pointing to a pathogenic role of S100B-RAGE interactions in multiple sclerosis [313]. The different effects exerted by S100B at low and high doses on nervous cells likely depend on the level of RAGE expression, different intensities of RAGE activation, the duration of RAGE stimulation and/or different extents of S100B-induced upregulation of RAGE expression in neurons, astrocytes and microglia.
Fig. (11).
Schematic representation of proposed extracellular effects of S100B on neurons microglia, astrocytes, myoblasts, VSMCs, cardiomyocytes, and peripheral nerves. (A) In normal physiological conditions S100B secreted by astrocytes exerts trophic effects on neurons and modulates microglial activity by engaging RAGE. (B) When present in the brain extracellular space at high concentrations, S100B activates microglia and astrocytes thus participating in the inflammatory response and is toxic to neurons by excessively stimulating RAGE. (C) In acute peripheral nerve injury S100B released from activated Schwann cells promotes macrophages and Schwann cell migration and the release of trophic factors via RAGE engagement thereby participating in peripheral nerve regeneration. (D) At subnanomolar-nanomolar doses S100B stimulates myoblast proliferation and inhibits myoblast differentiation by enhancing bFGF/FGFR1 signaling. However, in low-density myoblast cultures and at the very beginning of skeletal muscle regeneration, S100B engages RAGE in activated muscle satellite cells thereby stimulating proliferation and activating the myogenic program. (E) At high doses S100B stimulates VSMC proliferation and secretion of IL-6 and MCP-1 from VSMCs via RAGE engagement thus potentially participating in atherogenesis. (F) At high doses S100B causes cardiomyocyte apoptosis via RAGE engagement.
S100B null mice exhibit enhanced spatial and fear memories as well as enhanced long-term potentiation (LTP) in the hippocampal CA1 region, and perfusion of hippocampal slices with S100B reverses the levels of LTP to those of the wild-type slices [314]. This suggests that extracellular S100B might play a role as a regulator of synaptic plasticity, although the molecular mechanism underlying this activity remains to be elucidated. S100B appears to have an important role in the mechanism of action of the antidepressant, fluoxetine. Fluoxetine-treated serotoninergic neurons secrete S100B which acts on noradrenergic neurons thereby reducing the otherwise elevated microRNA-16, which blocks serotonin transporter translation therein [152]. Thus, secreted S100B induces noradrenergic neurons to acquire properties of serotoninergic neurons, thus mediating effects of fluoxetine and representing a potent endogenous antidepressant. However, no information is available concerning the mechanism whereby fluoxetine induces serotoninergic neurons to express and secrete S100B or the mechanism whereby S100B reduces microRNA-16 levels in noradrenergic neurons. Overall, whereas S100B was localized to in vitro cultured serotoninergic neurons [152], no evidence has been provided that that serotoninergic neurons do express S100B following in vivo treatment with fluoxetine. RAGE ligation might be implicated because S100B reduces microRNA-16 levels in monocytes in a RAGE-dependent manner [315]. While it is not known whether S100B null or RAGE null mice are refractory to fluoxetine, the enhanced spatial and fear memories reported in S100B null mice [314] might be consistent with the proposed role of S100B as a transducer of fluoxetine antidepressant action. The neurotoxin 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine (MPTP), which causes neurological and pathological changes comparable to those observed in Parkinson's disease [316], increases S100B expression in astrocytes in vivo [317], and the culture medium of MPTP-treated astrocytes reduces PC12 neuronal cell viability, an effect that can be counteracted by an S100B neutralizing antibody [318]. Reduced PC12 neuronal cell viability under these conditions has been thus attributed to enhanced release of S100B by astrocytes and S100B-induced cell death. However, treatment of MPTP-treated mice with the antiepileptic drug, zonisamide, which improves Parkinson's disease symptoms, has been shown to result in enhanced S100B expression in astrocytes and suggested to ameliorate clinical signs of the disease via augmented secretion of the neurotrophic S100B [319]. Thus, it seems that at the levels found in the brain extracellular space in normal physiological conditions, S100B is trophic to neural cells. However, in a background of inflammation and/or neurodegenerative disorders, which is accompanied by enhanced expression levels of S100B and the S100B receptor, RAGE, in neural and inflammatory cells [7,320-322], S100B acts synergistically with proinflammatory cytokines and, at higher concentrations, behaves itself as a cytokine, amplifying and perpetuating inflammation and causing oxidative damage to neurons.
Outside the central nervous system, S100B RAGE-dependently promotes Schwann cell migration during the course of repair of injured peripheral nerves through the induction of the expression of thioredoxin interacting protein and the consequent activation of p38 MAPK, CREB and NF-κB in Schwann cells [323] (Fig. 11C). Indeed, reduction of RAGE activity in acutely damaged peripheral nerves results in suppression of anatomical regeneration and functional recovery [324,325], supporting a role of RAGE in neurite outgrowth. Also, S100B released from injured skeletal muscle tissue is a potent stimulator of myoblast proliferation and inhibitor of myoblast differentiation via the enhancement of bFGF/FGFR1 signaling and blockade of RAGE's promyogenic signaling provided bFGF is present [11,326,327] (Fig. 11D). However, S100B effects on myoblasts are strongly dependent on cell density because in low-density myoblast cultures, and at early stage of low-density myoblast differentiation, it engages RAGE but not bFGF/FGFR1, thereby simultaneously stimulating proliferation and activating the myogenic program [328]. Thus, released S100B might contribute to the regeneration of injured skeletal muscle tissue by expanding the myoblast population at the site of injury and preventing precocious myoblast differentiation. Moreover, upon forming complexes with TLR-2 ligands, (low) S100B inhibits TLR-2 activation via RAGE, through a paracrine epithelial cell/neutrophil circuit that restrains pathogen-induced inflammation; however, upon binding to nucleic acids, S100B activates intracellular TLR-3/9 eventually resolving danger-induced inflammation via transcriptional inhibition of S100B [12]. Yet, at high doses, S100B engages RAGE on monocytes, promoting activation [329] and upregulation of microRNA-16 [315]. In activation of RAGE in autoimmune diabetes [330], S100B increases cyclooxygenase 2 expression in human pancreatic islets [331], and activates oxidant stress and inflammatory pathways in VSMCs [332] (Fig. 11E). S100B engages RAGE in endothelial cells thereby activating NF-κB transcriptional activity, increasing expression of vascular cell adhesion molecule-1 and inducing monocyte chemoattractant protein-1 and RAGE transcripts and eliminating sodium nitroprusside-potentiated vasodilatation in response to acetylcholine in endothelial dysfunction in type II diabetic (Leprdb) mice; also, S100B enhances the interaction of RAGE with the leukocyte β2-integrin Mac-1 thus potentially increasing leukocyte adhesion to endothelial cells (for review see [7]). At high doses S100B enhances eosinophil survival in a RAGE-dependent manner, an event that might contribute to the role of eosinophils in wound repair and the clearance of damaged cells [333].
S100B also stimulates RAGE-mediated proliferation of T cells directed to the acetylcholine receptor and upregulation of cyclo-oxygenase 2 expression in splenocytes, implicating the S100B/RAGE axis in the pathogenesis of myasthenia gravis [334]. S100B is expressed in, and secreted by CD8+ T lymphocytes and NK cells [153], thus potentially participating in the innate immune response, as outlined above, and in the adaptive immune response via RAGE engagement. The disparate effects of S100B [12,334], i.e. resolution and exacerbation of inflammation by the S100B/RAGE axis, respectively, are likely to depend on different types of responding cells coming into play in two conditions and differences in S100B doses and levels of RAGE expression. For example, for TLR-2 signaling, there is no role associated with myasthenia gravis whereas this has a critical role in inflammation caused by fungal infections. Thus, effects of the activity of the S100B/RAGE axis are likely to be strongly context-dependent. As mentioned earlier, S100B is induced by catecholamines in cardiomyocytes surviving an infarct, thereby modulating left ventricular remodeling [14,335]. However, S100B can be released by injured cardiomyocytes and at relatively high doses it causes RAGE-dependent cardiomyocyte apoptosis [336] (Fig. 11F) and contributes to scar formation in infarcted myocardium by stimulating VEGF secretion by cardiomyocytes, again in a RAGE-dependent manner [337]. S100B-RAGE interactions have important roles in vascular smooth muscle cell proliferation in diabetes [338] and in neovascular macular disease [166].
S100G
No extracellular function has been reported for S100G.
S100P
S100P can mediate tumor growth, drug resistance and metastasis through RAGE binding on cancer cells [339].
S100Z
No extracellular function has been reported for S100Z.
S100 PROTEINS AS DISEASE MARKERS
In acute inflammation, there are generally elevated systemic levels of S100A8, S100A9 and/or S100A12, likely released from extravasating neutrophils and activated macrophages that dominate these lesions and these proteins are suggested as non-specific markers of phagocyte activation [340]. Importantly, S100A8/A9 and S100A12 are not disease-specific, and would likely not discriminate one particular condition if there were an underlying second pathology. Incorporation of S100A8/A9 and S100A12 levels with other particular disease markers may increase effective use for diagnosis. However there are limitations concerning the diagnostic use of these markers, as some results are conflicting [336], and for some applications, more robust assessment, with large patient cohorts is required. High circulating levels of S100A8/A9 and S100A12 are found in patients suffering from numerous infections or acute and chronic inflammatory disorders, various types of tumors, obesity, and Alzheimer's disease [262,341,342]. Secretions, including tears, saliva, sputum, cyst fluid, cerebrospinal fluid, synovial fluid, urine and feces may contain these proteins in particular circumstances. Concentrations of S100 proteins frequently correlate with disease course and/or severity, and may correlate with clinical scores and decline following therapy. Conditions associated with elevated S100A8/A9 and S100A12 levels in the circulation and secretions, and the use of these S100 proteins as clinical markers of inflammation are reviewed in [143,340].
Among the most recent applications is the measurement of S100A8/A9 or S100A12 in the circulation and/or feces from patients with gastrointestinal inflammation. The usefulness of a noninvasive fecal test is particularly useful for pediatric cases and levels may be used as markers for differential diagnosis, monitoring activity, or disease relapse in inflammatory bowel disease (IBD) [341,344]. Similarly, in cardiovascular disease, increased plasma levels of S100A8/A9 may predict cardiovascular events in humans [345]. When measured with myeloperoxidase and C-reactive protein, elevated levels predict all-cause mortality from acute myocardial infarction with moderate accuracy [346]. Others showed that the risk of a recurrent cardiovascular event increased with each increasing S100A8/S100A9; patients with the highest levels had a 2.0-fold increased risk [347]. However, in patients with stable coronary artery disease, S100A8/S100A9 levels did not associate with any other cardiovascular disease risk factor, and serum levels did not differ from normal subjects [348]. S100A8/S100A9 levels are also elevated in patients with inflammatory arthritides [143], and may be a predictive marker of clinical improvement following treatment of patients in the early phase of rheumatoid arthritis [349]. High amounts of S100A8, S100A9 and S100A12 in synovial fluid distinguished rheumatoid arthritis from miscellaneous inflammatory arthritides with high accuracy [350] and S100A8/S100A9 may be a prognostic biomarker for erosive disease in patients with rheumatoid arthritis [351].
S100A12 may be a useful marker of some lung disorders. We found that elevated sputum levels may be useful to assess eosinophilic asthma [284]. High concentrations of MMP-7, ICAM-1, IL-8, VCAM-1, and S100A12 predicted poor overall survival of patients with idiopathic pulmonary fibrosis [352] and S100A12 is useful as an early marker of acute, early-stage lung injury in patients with sepsis [353,354].
The use of other S100 proteins as markers is only emerging, particularly from proteome studies and gene arrays. Some that are elevated in certain cancers may be useful. For example, S100A9 is proposed as a good marker for identifying myeloid-derived suppressor cells in the circulation of patients with certain cancers [355]; overexpression of S100A9 is associated with a poor prognosis in non-small cell lung cancer patients and may identify patients at high risk, even at an early pathological stage [356]. High expression of S100A11 in pancreatic adenocarcinoma might be a significant tumor marker and an unfavorable predictor for prognosis of patients who have undergone surgical resection [357]. S100A4 acts as a metastasis inducer; high levels in primary colon, rectal, and gastric tumors are prognostic for metastasis and correlate with reduced patient survival [358], elevated amounts of nuclear S100A7 is associated with poor prognosis in head and neck cancer [359] and S100A7 is associated with the worst prognosis in estrogen receptor α-negative invasive breast cancers [50].
S100B is normally found in pM amounts in human serum [for review see Refs. 141,360], saliva [361,362], urine [363,364], amniotic fluid [365] and milk [366]. Serum levels of S100B are elevated after central nervous system injury such as stroke, subarachnoid hemorrhage and brain trauma, generally peaking 2-3 days after injury, and correlate positively with patient outcome [141-144,367-370]. However, in accordance with the notion that S100B may be released from non-nervous cells as well [7], elevated serum S100B levels are also found in several non-neurological clinical conditions such as cardiac ischemia, cardiomyopathy and extracerebral infections, and in patients who have undergone cardiothoracic surgical interventions or had a trauma without brain injury [371-378]. Schizophrenia [131,157,379,380], depressive/bipolar disorders [381,382] and obesity [383] also are associated with high serum S100B levels, but there is uncertainty as to whether nervous or non-nervous cells are the source of S100B in these conditions. High S100B levels are also found in the cerebrospinal fluid of patients with multiple sclerosis during the acute phase of exacerbation [384] and in bacterial meningitis [385]. Moreover, elevated S100B levels in amniotic fluid correlate positively with the occurrence of central nervous system malformations and damage [386] and are found in trisomy 21-complicated pregnancies [387,388]. Lastly, as mentioned earlier serum levels of S100B are of prognostic value in patients with cutaneous melanoma [105] and breast cancer [132].
THERAPEUTIC CONSIDERATIONS FOR THE S100 PROTEIN FAMILY
In addition to the use of S100 proteins as diagnostic markers, there is evidence that deregulation of S100 proteins can contribute to numerous disease states including cancer. With this in mind, regulating S100-dependent biology is becoming more widespread. The most straightforward means to achieve this goal in the clinic is to inhibit S100 proteins directly with small molecule inhibitors. For this approach, typically a combination of computer aided drug design (CADD), high-throughput screening, structural biology, medicinal chemistry, and in vivo biology and drug testing approaches are employed. Structure-based design is particularly useful since target specificity issues can be considered, and many high-resolution 3D structures and dynamic data for S100 complexes are available [20,389-396]. Having structural and dynamic information at atomic resolution is very helpful because of the somewhat difficult hurdle of inhibiting protein-protein interactions involving S100s [392-395,397,398]. One promising approach for this is to use CADD, NMR, X-ray crystallography and medicinal chemistry approaches to identify and then simultaneously block adjacent small molecule binding sites with a single engineered molecule [399,400]. As with any drug-design program, there is also a need to obtain physiological data at an early stage in the process to help determine whether a compound or series of compounds induce off-target effects and/or cause other unanticipated toxicities. This is particularly important for S100 inhibitors since there are over 20 structurally similar proteins, and they regulate many physiological pathways in a cell-specific manner [20,401].
Small molecule inhibitors have been reported for numerous S100 family members, some of which are in human clinical trials. For example, the anti-allergic drug cromolyn disrupts the S100P-RAGE interaction and reduces pancreatic tumor formation in animal models [402]. Cromolyn also binds other S100 family members (S100A1, S100A12 and S100A13), but its effects on the interaction of these proteins have not yet been fully investigated. Inhibitors of the S100A10-annexin A2 interaction are also available with clinical uses predicted for angiogenesis and cancer metastasis [403]. With the goal of treating several cognitive disorders, small molecules that block S100A1 are also being pursued with drugs such as pentamidine, propranolol, and others S100A1 inhibitors (termed SA1iXs); although, for safety purposes, it will be important to consider the role S100A1 has in normal muscle contraction [396]. For S100A4, several phenothiazines including trifluoperazine and prochlorperazine were shown to disrupt the S100A4/myosin-IIA interaction by sequestering S100A4 via small molecule-induced oligomerization [40,404]. It is clear that these and/or other S100A4 inhibitors can be developed further and may have promise for several metastatic cancers [40,405]. In another study, S100A4 was shown to bind to anti-allergic drugs and a modified version of azaxanthone is being tested clinical trials for treatment of metastatic disease [405,406]. Likewise, a role for S100A4 has been suggested for the regulation of MMP and tissue inhibitors of MMPs activities, so in addition to cancer, pathological consequences of elevated S100A4 may also be important to block in patients with arthritis [407]. For S100B, a series of inhibitors were discovered (termed SBiXs) for treating malignant melanoma [107,408]. This work has included repurposing drugs such as pentamidine [408] and chlorpromazine [409,410] and modified versions of these SBiXs for melanoma treatment. Currently, the goal of this work is to use structure-based drug design approaches to engineer improved and highly specific S100B inhibitors, which have higher efficacy and fewer side effects than those currently being tested. Thus SBiXs that restore p53 function by specifically inhibiting S100B, as found for S100B siRNA, is a major goal [107,408]. In this regard, human and veterinary clinical trials are underway with two such SBiXs as potential melanoma therapeutics. However, as with all biological targets, it is often necessary to consider how populations of cells become resistant to drug treatment. In the case of blocking the S100B-p53 protein-protein interaction, this is necessary because a negative feedback loop is initiated when p53 levels are restored since the gene for S100B itself is up regulated by p53 [411]. Thus, cells that survive the initial SBiX treatment predictably have extremely high levels of the S100B protein expressed and require higher secondary doses of drug to induce apoptosis [unpublished data, Weber D.J. et al.].
Another therapeutic strategy is to control S100 expression. It is well established that the genes for several S100 proteins are associated with cell differentiation, malignant transformation, and cell cycle growth (i.e. S100B, S100A6, and others), and that their expression levels are varied according to various growth- and growth-inhibitory conditions, often in a cell cycle dependent manner [17]. Thus, treatments with general cell growth-inhibitors, such as the topoisomerase II inhibitor VP-16 or phorbol 12-myristate 13-acetate often have varying effects on levels of specific S100 proteins and in a cell-specific manner [412]. Therefore, to regulating specific S100s in particular pathological cell types, screens for inhibitors that down-regulate the activation of a known promoter is typically used. For example, in a screen monitoring S100A4 expression, calcimycin was identified as a down-regulator of the S100A4 promoter [413,414]. Similarly expression of S100B in melanoma is high and problematic, and it was found that one regulator of S100B expression is the HOXC11 protein. Thus, treatment with the Src/Abl inhibitor, dasatinib, reduced the HOXC11-SRC-1 interaction and prevented recruitment of HOXC11 to the S100B promoter, so the mRNA and protein levels for S100B were both reduced, as was the growth and migratory properties of drug-treated MeWo melanoma cells. Thus, profiling patients for HOXC11 and S100B levels has the potential to increase the efficacy of dasatinib treatment in melanoma [415]. It is also noteworthy that the effect of specific drugs can be tissue-specific, particularly for regulation of the S100A2 gene, which is thought to suppress growth in some cell types (i.e. breast) and accelerate growth in others (i.e. skin). In keratinocytes, S100A2 mRNA levels were 5-fold induced after organ culture with its induction blocked by the drug PD153035, a specific inhibitor of EGF receptor tyrosine kinase activity. These and other studies in immortalized keratinocytes demonstrated that EGF receptor activation selectively upregulates S100A2, which is now thought to be an important mediator of regenerative epidermal hyperplasia [416].
Modulating S100 proteins is not limited to activities within the cell. For example, one major source of extracellular S100B are astrocytes, which sense, integrate, and respond to stimuli generated by neurons or neural injury, and this biology can involve gap junction (GJ) communication resulting in neurite growth, glial proliferation, and neuronal survival, at low levels of S100B. Interestingly, blocking GJ communication with specific inhibitors was found to stimulate S100B secretion in astrocyte cultures and acute hippocampal slices with one GJ blocker, carbenoxolone, shown to induce a fast and persistent increase in S100B secretion. Physiologically, a local GJ closure associated with release of low levels of S100B is postulated to be a mechanism necessary to limit the extension of lesion and increase the chances of neuronal cell survival [417]. However, elevated S100B levels within the brain demonstrate pathological effects because of its specific localization and selective overexpression in Alzheimer's disease [7]. Such an effect is thought to result from S100B-dependent activation of NO synthase to produce excessive NO [300-302,420-422]. Such a scenario can lead to astrocytic and neuronal cell death as found for astrocyte-neuron co-cultures [421]. Consistent with this hypothesis, block of this pathological effect was observed using specific NO synthase inhibitors and or via specific S100B antibodies, representing yet another therapeutic strategy to combat elevated S100B found in neurodegenerative cells associated with Alzheimer's and Parkinson's disease [422]. Clearly, though, such extracellular effects of S100s are not limited to the brain since S100s are involved in a in a wide array of important biological processes, such as angiogenesis, cell differentiation, and tumor growth. For example, a direct inhibitor of the S100A13-FGF1 complex, amlexanox, was shown to block the release of S100A13 and FGF1 resulting in the blocking of the angiogenic and mitogenic activities of FGF1 [423].
Another long-range goal is to regulate gene products via gene therapy approaches and or by up- or down-regulating specific micro-inhibitor RNA constructs (mIRs). For example, when an S100 is thought to be important for tumor suppression (i.e. S100A2 in breast) or when it is under-expressed or missing altogether (i.e. S100A1 in myopathies), gene therapy approaches have been considered. In one case, a conditionally replicative adenovirus (Ad/SA) for S100A2 was examined for its anti-tumor activity in vitro and in vivo for non-small-cell lung carcinoma [424]. In two EGFR-activated tumor xenograft animal models, Ad/SA exhibited potent anti-tumor activity, whereas cetuximab, an EGFR-targeting anticancer drug, was active transiently or ineffective. Combined treatment with cetuximab or cisplatin plus Ad/SA resulted in enhanced anti-tumor activity. Collectively, these results demonstrated that the S100A2 promoter-driven adenovirus could be a potent inhibitor of cancers such as non-small-cell lung carcinoma [424]. Likewise, knockout studies indicate that S100A1 is important for normal function of both heart and skeletal muscle, so gene therapy approaches have also been considered by many groups for the introduction of S100A1 for the treatment of myopathies lacking sufficient levels of S100A1 [425]. Likewise, using pharmacological and behavioral data, miR-16 was found to contribute positively to the therapeutic action of selective serotonin reuptake inhibitors (SSRI) via S100B for treating depression and anxiety [152], so there is also therapeutic potential for using mIRs and/or regulating their production specifically as yet another way to regulate S100 function in the future.
CONCLUDING REMARKS
With the exception of S100G which is a Ca2+-modulator protein implicated in the buffering of cytosolic Ca2+, all other members of the S100 protein family are Ca2+-sensor proteins involved in the regulation of several functions including cell proliferation and differentiation, tumor development, growth and metastasis, Ca2+ homeostasis, cell motility, apoptosis, redox, energy and glutathione metabolism, the inflammatory response, and transcription. However, these activities are not shared by all S100 members, each exhibiting cell-specific expression patterns and a peculiar set of intracellular target proteins which points to non-redundant functions of individual S100 protein members. In contrast to the universal Ca2+-sensor protein, calmodulin, which only acts intracellularly, certain S100 proteins are secreted (either constitutively or following specific stimuli) or released following tissue injury. Extracellular S100 proteins can act as growth factors involved in the regulation of tissue development and regeneration/repair, or as damage-associated molecular pattern factors (alarmins) involved in the pathogenesis of inflammatory (including autoimmune) and infectious diseases, allergy, tumorigenesis and metastasis, and microbial killing. Extracellular effects of S100 proteins are transduced by a battery of receptors such as RAGE, TLR-4, heparan sulfate proteoglycans and carboxylated N-glycans, G-protein-coupled receptors or scavenger receptors. Certain S100 proteins may also activate cell surface receptors in an indirect manner, i.e. by interacting with canonical receptor ligands to potentiate their signaling. S100 proteins are only expressed in vertebrates, and their expression and/or activities appear to be mechanistically linked to the refinement or fine tuning of cell-specific gene expression and responses to external stimuli. Intracellular and extracellular functions of S100 proteins are beginning to be described in detail, making these proteins less enigmatic than in the past. Cardiac function, tissue repair/regeneration, inflammation (including neuroinflammation), infection and cell growth and differentiation, are processes in which certain S100 proteins are active players. However, regulation of their expression requires more investigation; overexpression of particular S100s is associated with tumorigenesis, chronic inflammation and neurodegeneration, and elucidation of mechanisms controlling this may help identify molecular determinants of deranged cell/tissue functions and indicate new ways to target therapies. Also, the identification of highly specific inhibitors of individual S100 proteins and their usage in in vivo studies may establish functions of members of this protein family and represent therapeutic tools.
ACKNOWLEDGEMENTS
We wish to thank the reviewers for criticism, comments and suggestions. Supported by Ministero dell'Istruzione, dell'Università e della Ricerca - Italy (PRIN 2007LNKSYS, 2007AWZTHH_004, PRIN_2009 WBFZYM_002), Associazione per la Ricerca sul Cancro (Project 6021), Association Française contre les Myopathies (Project 12992) and Fondazione Cassa di Risparmio di Perugia (Project 2009.020.0021), NIH GM58888, NIH CA107331, and The National Health and Medical Research Council of Australia.
ABBREVIATIONS
- RyR
Ryanodine receptor
- TLR
Toll-like receptor
- NMR
Nuclear magnetic resonance
- PKC
Protein kinase C
- NMMHC IIA
Nonmuscle myosin heavy chain IIA
- MMPs
Matrix metalloproteinases
- IL
Interleukin
- ROS
Reactive oxygen species
- NO
Nitric oxide
- p38 MAPK
p38 mitogen-activated protein kinase
- TNF-α
Tumor necrosis factor-α
- tPa
Tissue plasminogen activator
- EGF
Epidermal growth factor
- VSMCs
Vascular smooth muscle cells
- IFN-γ
Interferon-γ
- FGF
Fibroblast growth factor
- IL
Interleukin
- ERK1/2
Extracellular signal-regulated kinase 1/2
- RAGE
receptor for advanced glycation end products
- NETs
neutrophil extracellular traps
- FGFR1
bFGF receptor 1
- EGFR
epidermal growth factor receptor
- LPS
Lipopolysaccharide
- LTP
Long-term potentiation
- MPTP
1-Methyl-4-phenyl 1,2,3,6 tetrahydropyridine
- SBiXs
S100B inhibitors
- GJ
Gap junction
- mIRs
Micro-inhibitor RNA constructs
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Carafoli E, Klee C. Calcium as a Cellular Regulator. Oxford University Press; New York: 1999. [Google Scholar]
- 2.Permyakov EA, Kretsinger RH. Cell signaling, beyond cytosolic calcium in eukaryotes. J Inorg Biochem. 2009;103:77–86. doi: 10.1016/j.jinorgbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;2001;33:637–8. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 4.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004;322:1111–22. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 5.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med. 2007;7:711–24. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Sinha M, Luxon BA, Bresnick AR, O'Connor KL. Integrin α6β4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem. 2009;284:1484–94. doi: 10.1074/jbc.M803997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donato R, Sorci G, Riuzzi F, et al. S100B's double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–22. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–66. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 9.Hsu K, Champaiboon C, Guenther BD, et al. Anti-infective protective properties of S100 calgranulins. Antiinflamm Antiallergy Agents Med Chem. 2009;8:290–305. doi: 10.2174/187152309789838975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TH, Kim HI, Soung YH, Shaw LA, Chung J. Integrin (α6β4) signals through Src to increase expression of S100A4, a metastasis-promoting factor: implications for cancer cell invasion. Mol Cancer Res. 2009;7:1605–12. doi: 10.1158/1541-7786.MCR-09-0102. [DOI] [PubMed] [Google Scholar]
- 11.Riuzzi F, Sorci G, Donato R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J Cell Sci. 2011;124:2389–400. doi: 10.1242/jcs.084491. [DOI] [PubMed] [Google Scholar]
- 12.Sorci G, Giovannini G, Riuzzi F, et al. The danger signal S100B integrates pathogen- and danger-sensing pathways to restrain inflammation. PLoS Pathog. 2011;7:e1001315. doi: 10.1371/journal.ppat.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggers K, Sikora K, Lorenz M, et al. RAGE-dependent regulation of calcium-binding proteins S100A8 and S100A9 in human THP-1. Exp Clin Endocrinol Diabetes. 2011;119:353–7. doi: 10.1055/s-0030-1268426. [DOI] [PubMed] [Google Scholar]
- 14.Tsoporis JN, Marks A, Haddad A, Dawood F, Liu PP, Parker TG. S100B expression modulates left ventricular remodeling after myocardial infarction in mice. Circulation. 2005;111:598–606. doi: 10.1161/01.CIR.0000154554.65287.F5. [DOI] [PubMed] [Google Scholar]
- 15.Moews PC, Kretsinger RH. Refinement of the structure of carp muscle calcium-binding parvalbumin by model building and difference Fourier analysis. J Mol Biol. 1975;91:201–25. doi: 10.1016/0022-2836(75)90160-6. [DOI] [PubMed] [Google Scholar]
- 16.Strynadka NC, James MN. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–98. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 17.Kligman D, Hilt DC. The S100 protein family. Trends Biochem Sci. 1988;13:437–43. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- 18.Yap KL, Ames JB, Swindells MB, Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499–507. doi: 10.1002/(sici)1097-0134(19991115)37:3<499::aid-prot17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer DB, Wright Sadosky P, Weber DJ. Molecular mechanisms of S100-target protein interactions. Microsc Res Tech. 2003;60:552–9. doi: 10.1002/jemt.10297. [DOI] [PubMed] [Google Scholar]
- 20.Zimmer DB, Weber DJ. The calcium-dependent interaction of S100B with its protein targets. Cardiovasc Psychiatry Neurol. 2010 doi: 10.1155/2010/728052. doi.10.1155/2010/728052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charpentier TH, Thompson LE, Liriano MA, et al. The effects of CapZ peptide (TRTK-12) binding to S100B-Ca2+ as examined by NMR and X-ray crystallography. J Mol Biol. 2010;396:1227–43. doi: 10.1016/j.jmb.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donato R. Perspectives in S-100 protein biology. Cell Calcium. 1991;12:713–26. doi: 10.1016/0143-4160(91)90040-l. [DOI] [PubMed] [Google Scholar]
- 23.Heizmann CW. The multifunctional S100 protein family. Meth Mol Biol. 2002;172:69–80. doi: 10.1385/1-59259-183-3:069. [DOI] [PubMed] [Google Scholar]
- 24.Prosser BL, Wright NT, Hernãndez-Ochoa EO, et al. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem. 2008;283:5046–57. doi: 10.1074/jbc.M709231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright NT, Prosser BL, Varney KM, Zimmer DB, Schneider MF, Weber DJ. S100A1 and calmodulin compete for the same binding site on ryanodine receptor. J Biol Chem. 2008;283:26676–83. doi: 10.1074/jbc.M804432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 27.Kiewitz R, Lyons GE. Schäfer BW, Heizmann CW. Transcriptional regulation of S100A1 and expression during mouse heart development. Biochim Biophys Acta. 2000;1498:207–19. doi: 10.1016/s0167-4889(00)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Most P, Seifert H, Gao E, et al. Cardiac S100A1protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–68. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 29.Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:525–37. doi: 10.1007/s12265-010-9211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Völkers M, Rohde D, Goodman C, Most P. S100A1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J Biomed Biotechnol. 2010;2010:178614. doi: 10.1155/2010/178614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Most P, Pleger ST, Völkers M, et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–63. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prosser BL, Hernández-Ochoa EO, Zimmer DB, Schneider MF. The Qy component of intra-membrane charge movement is present in mammalian muscle fibres, but suppressed in the absence of S100A1. J Physiol. 2009;587:4523–41. doi: 10.1113/jphysiol.2009.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf S, Haase-Kohn C, Pietzsch J. S100A2 in cancerogenesis: a friend or a foe? Amino Acids. 2010;41:849–61. doi: 10.1007/s00726-010-0623-2. [DOI] [PubMed] [Google Scholar]
- 34.van Dieck J, Teufel DP, Jaulent AM, et al. Posttranslational modifications affect the interaction of S100 proteins with tumor suppressor p53. J Mol Biol. 2009;394:922–30. doi: 10.1016/j.jmb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Kizawa K, Takahara H, Troxler H, Kleinert P, Mochida U, Heizmann CW. Specific citrullination causes assembly of a globular S100A3 homotetramer: a putative Ca2+ modulator matures human hair cuticle. J Biol Chem. 2008;283:5004–13. doi: 10.1074/jbc.M709357200. [DOI] [PubMed] [Google Scholar]
- 36.Kizawa K, Inoue T, Yamaguchi M, et al. Dissimilar effect of perming and bleaching treatments on cuticles: advanced hair damage model based on elution and oxidation of S100A3 protein. J Cosmet Sci. 2005;56:219–26. [PubMed] [Google Scholar]
- 37.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–80. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 38.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–35. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail TM, Fernig DG, Rudland PS, Terry CJ, Wang G, Barraclough R. The basic C-terminal amino acids of calcium-binding protein S100A4 promote metastasis. Carcinogenesis. 2008;29:2259–66. doi: 10.1093/carcin/bgn217. [DOI] [PubMed] [Google Scholar]
- 40.Malashkevich VN, Dulyaninova NG, Ramagopal UA, et al. Phenothiazines inhibit S100A4 function by inducing protein oligomerization. Proc Natl Acad Sci USA. 2010;107:8605–10. doi: 10.1073/pnas.0913660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li ZH, Dulyaninova NG, House RP, Almo SC, Bresnick AR. S100A4 regulates macrophage chemotaxis. Mol Biol Cell. 2010;21:2598–610. doi: 10.1091/mbc.E09-07-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein U, Arlt F, Walther W, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486–500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Stein U, Arlt F, Smith J, et al. Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13:131–44. doi: 10.1593/neo.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–8. [PubMed] [Google Scholar]
- 45.Hancq S, Salmon I, Brotchi J, et al. S100A5: a marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol. 2004;30:178–87. doi: 10.1046/j.0305-1846.2003.00525.x. [DOI] [PubMed] [Google Scholar]
- 46.Leśniak W, Słomnicki ŁP, Filipek A. S100A6 - new facts and features. Biochem Biophys Res Commun. 2009;390:1087–92. doi: 10.1016/j.bbrc.2009.10.150. [DOI] [PubMed] [Google Scholar]
- 47.Leśniak W, Filipek A, Donato R. S100a6. UCSD-Nature Molecule Pages. 2009 DOI: 10.1038/mp.a002122.01. [Google Scholar]
- 48.Słomnicki ŁP, Narrow B, Leśniak W. S100A6 binds p53 and affects its activity. Int J Biochem Cell Biol. 2009;41:784–90. doi: 10.1016/j.biocel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Tsoporis JN, Izhar S, Parker TG. Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-α. J Biol Chem. 2008;283:30174–83. doi: 10.1074/jbc.M805318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emberley ED, Niu Y, Curtis L, et al. The S100A7-c-Jun activation domain binding protein 1 pathway enhances prosurvival pathways in breast cancer. Cancer Res. 2005;65:5696–702. doi: 10.1158/0008-5472.CAN-04-3927. [DOI] [PubMed] [Google Scholar]
- 51.West NR, Watson PH. S100A7 (psoriasin) is induced by the proinflammatory cytokines oncostatin-M and interleukin-6 in human breast cancer. Oncogene. 2010;29:2083–92. doi: 10.1038/onc.2009.488. [DOI] [PubMed] [Google Scholar]
- 52.Deol YS, Nasser MW, Yu L, Zou X, Ganju RK. Tumor-suppressive effects of psoriasin (S100A7) are mediated through the β-catenin/T cell factor 4 protein pathway in estrogen receptor-positive breast cancer cells. J Biol Chem. 2011;286:44845–54. doi: 10.1074/jbc.M111.225466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passey RJ, Williams E, Lichanska AM, et al. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–16. [PubMed] [Google Scholar]
- 54.Grimbaldeston MA, Geczy CL, Tedla N, Finlay-Jones J, Hart PH. S100A8 induction in keratinocytes by UVA-irradiation is dependent on reactive oxygen intermediates. J Invest Dermatol. 2003;121:1168–74. doi: 10.1046/j.1523-1747.2003.12561.x. [DOI] [PubMed] [Google Scholar]
- 55.Lagasse E, Weissman IL. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992;79:1907–15. [PubMed] [Google Scholar]
- 56.Rosenberger S, Thorey IS, Werner S, Boukamp P. A novel regulator of telomerase. S100A8 mediates differentiation-dependent and calcium-induced inhibition of telomerase activity in the human epidermal keratinocyte line HaCaT. J Biol Chem. 2007;282:6126–35. doi: 10.1074/jbc.M610529200. [DOI] [PubMed] [Google Scholar]
- 57.Lim SY, Raftery M, Cai H, et al. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181:5627–36. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- 58.Vogl T, Ludwig S, Goebeler M, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104:4260–68. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 59.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 61.Lominadze G, Rane MJ, Merchant M, Cai J, Ward RA, McLeish KR. Myeloid-related protein-14 is a p38 MAPK substrate in human neutrophils. J Immunol. 2005;174:7257–67. doi: 10.4049/jimmunol.174.11.7257. [DOI] [PubMed] [Google Scholar]
- 62.Mc Neill E, Conway SJ, Roderick HL, Bootman MD, Hogg N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium. 2007;41:107–21. doi: 10.1016/j.ceca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Lim SY, Raftery MJ, Goyette J, Geczy CL. S-glutathionylation regulates inflammatory activities of S100A9. J Biol Chem. 2010;285:14377–88. doi: 10.1074/jbc.M109.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Zhang F, Lin M, Liu J. Induction of S100A9 gene expression by cytokine oncostatin M in breast cancer cells through the STAT3 signaling cascade. Breast Cancer Res Treat. 2004;87:123–34. doi: 10.1023/B:BREA.0000041594.36418.f6. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Chen H, Ding F, et al. A novel p53 target gene, S100A9, induces p53-dependent cellular apoptosis and mediates the p53 apoptosis pathway. Biochem J. 2009;422:63–372. doi: 10.1042/BJ20090465. [DOI] [PubMed] [Google Scholar]
- 66.Inaba H, Hokamura K, Nakano K, et al. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett. 2009;583:128–34. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 67.Murao S, Collart FR, Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989;264:8356–60. [PubMed] [Google Scholar]
- 68.Averill MM, Barnhart S, Becker L, et al. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123:1216–26. doi: 10.1161/CIRCULATIONAHA.110.985523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuwayama A, Kuruto R, Horie N, Takeishi K, Nozawa R. Appearance of nuclear factors that interact with genes for myeloid calcium binding proteins (MRP-8 and MRP-14) in differentiated HL-60 cells. Blood. 1993;81:3116–21. [PubMed] [Google Scholar]
- 70.Siegenthaler G, Roulin K, Chatellard-Gruaz D, et al. A heterocomplex formed by the calcium-binding proteins MRP8 (S100A8) and MRP14 (S100A9) binds unsaturated fatty acids with high affinity. J Biol Chem. 1997;272:9371–77. doi: 10.1074/jbc.272.14.9371. [DOI] [PubMed] [Google Scholar]
- 71.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–9. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 72.Steinckwich N, Schenten V, Melchior C, Bréchard S, Tschirhart EJ. An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcyR-mediated phagosomal oxidative activity. J Immunol. 2011;186:2182–91. doi: 10.4049/jimmunol.1001338. [DOI] [PubMed] [Google Scholar]
- 73.Nemeth J, Stein I, Haag D, et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 2009;50:1251–62. doi: 10.1002/hep.23099. [DOI] [PubMed] [Google Scholar]
- 74.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 75.Sunderkotter CH, Tomimori-Yamashita J, Nix V, et al. High expression of myeloid-related proteins 8 and 14 characterizes an inflammatorily active but ineffective response of macrophages during leprosy. Immunology. 2004;111:472–80. doi: 10.1111/j.0019-2805.2004.01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaia AA, Sappington KJ, Nisapakultorn K, et al. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009;2:43–53. doi: 10.1038/mi.2008.63. [DOI] [PubMed] [Google Scholar]
- 77.Arai K, Takano S, Teratani T, Ito Y, Yamada T, Nozawa R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets. 2008;8:243–52. doi: 10.2174/156800908784533445. [DOI] [PubMed] [Google Scholar]
- 78.Bode G, Lüken A, Kerkhoff C, Roth J, Ludwig S, Nacken W. Interaction between S100A8/A9 and annexin A6 is involved in the calcium-induced cell surface exposition of S100A8/A9. J Biol Chem. 2008;283:31776–84. doi: 10.1074/jbc.M803908200. [DOI] [PubMed] [Google Scholar]
- 79.Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J. 1995;309:419–24. doi: 10.1042/bj3090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch. 2008;455:575–82. doi: 10.1007/s00424-007-0313-4. [DOI] [PubMed] [Google Scholar]
- 82.Rezvanpour A, Santamaria-Kisiel L, Shaw GS. The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair. J Biol Chem. 2011;286:40174–83. doi: 10.1074/jbc.M111.244038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warner-Schmidt JL, Chen EY, Zhang X, et al. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry. 2010;68:528–35. doi: 10.1016/j.biopsych.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakaguchi M, Miyazaki M, Sonegawa H, et al. PKC mediates TGF-induced growth inhibition of human keratinocytes via phosphorylation of S100C/A11. J Cell Biol. 2004;164:979–84. doi: 10.1083/jcb.200312041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murzik U, Hemmerich P, Weidtkamp-Peters S, et al. Rad54B targeting to DNA double-strand break repair sites requires complex formation with S100A11. Mol Biol Cell. 2008;19:2926–35. doi: 10.1091/mbc.E07-11-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakaguchi M, Sonegawa H, Murata H, et al. S100A11, a dual mediator for growth regulation of human keratinocytes. Mol Biol Cell. 2008;19:78–85. doi: 10.1091/mbc.E07-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2010;41:821–42. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 88.Vogl T, Pröpper C, Hartmann M, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–6. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 89.Hatakeyama T, Okada M, Shimamoto S, Kubota Y, Kobayashi R. Identification of intracellular target proteins of the calcium-signaling protein S100A12. Eur J Biochem. 2004;271:3765–75. doi: 10.1111/j.1432-1033.2004.04318.x. [DOI] [PubMed] [Google Scholar]
- 90.Hitomi J, Kimura T, Kusumi E, et al. Novel S100 proteins in human esophageal epithelial cells: CAAF1 expression is associated with cell growth arrest. Arch Histol Cytol. 1998;61:163–78. doi: 10.1679/aohc.61.163. [DOI] [PubMed] [Google Scholar]
- 91.Hofmann Bowman M, Wilk J, Heydemann A, et al. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ. Res. 2010;106:145–54. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hofmann Bowman MA, Gawdzik J, Bukhari U, et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–44. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofmann Bowman MA, Heydemann A, Gawdzik J, Shilling RA, Camoretti-Mercado B. Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation. Clin Exp Allergy. 2011;41:878–89. doi: 10.1111/j.1365-2222.2011.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prudovsky I, Donato R. S100a13. UCSD-Nature Molecule Pages. 2009 DOI: 10.1038/mp.a002115.01. [Google Scholar]
- 95.Chen H, Yu D, Luo A, et al. Functional role of S100A14 genetic variants and their association with esophageal squamous cell carcinoma. Cancer Res. 2009;69:3451–7. doi: 10.1158/0008-5472.CAN-08-4231. [DOI] [PubMed] [Google Scholar]
- 96.Sapkota D, Bruland O, Costea DE, Haugen H, Vasstrand EN, Ibrahim SO. S100A14 regulates the invasive potential of oral squamous cell carcinoma derived cell-lines in vitro by modulating expression of matrix metalloproteinases, MMP1 and MMP9. Eur J Cancer. 2011;47:600–10. doi: 10.1016/j.ejca.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 97.Chen H, Yuan Y, Zhang C, et al. Involvement of S100A14 protein in cell invasion by affecting expression and function of matrix metalloproteinase (MMP)-2 via p53-dependent transcriptional regulation. J Biol Chem. 2012;287:17109–19. doi: 10.1074/jbc.M111.326975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids. 2010;41:789–96. doi: 10.1007/s00726-010-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem. 2006;281:38905–17. doi: 10.1074/jbc.M605798200. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Zhang R, Xin J, et al. Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology. 2011;152:903–11. doi: 10.1210/en.2010-1059. [DOI] [PubMed] [Google Scholar]
- 101.Hachem S, Laurenson AS, Hugnot JP, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev Biol. 2007;7:17. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raponi E, Agenes F, Delphin C, et al. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–77. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saito T, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep. 2007;8:504–9. doi: 10.1038/sj.embor.7400934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Hamada J, Nishimoto A, et al. HOXC6 and HOXC11 increase transcription of S100β gene in BrdU-induced in vitro differentiation of GOTO neuroblastoma cells into Schwannian cells. J Cell Mol Med. 2007;11:299–306. doi: 10.1111/j.1582-4934.2007.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mocellin S, Zavagno G, Nitti D. The prognostic value of serum S100B in patients with cutaneous melanoma: a meta-analysis. Int J Cancer. 2008;123:2370–6. doi: 10.1002/ijc.23794. [DOI] [PubMed] [Google Scholar]
- 106.Brozzi F, Arcuri C, Giambanco I, Donato R. S100B protein regulates astrocyte shape and migration via interaction with Src kinase: implications for astrocyte development, activation and tumor growth. J Biol Chem. 2009;284:8797–811. doi: 10.1074/jbc.M805897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin J, Yang Q, Wilder PT, Carrier F, Weber DJ. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J Biol Chem. 2010;285:27487–98. doi: 10.1074/jbc.M110.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tubaro C, Arcuri C, Giambanco I, Donato R. S100B protein in myoblasts modulates myogenic differentiation via NF-κB-dependent inhibition of MyoD expression. J Cell Physiol. 2010;223:270–82. doi: 10.1002/jcp.22035. [DOI] [PubMed] [Google Scholar]
- 109.Beccafico S, Riuzzi F, Puglielli C, et al. Human muscle satellite cells show age-related differential expression of S100B protein and RAGE. Age. 2011;33:523–41. doi: 10.1007/s11357-010-9197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tubaro C, Arcuri C, Giambanco I, Donato R. S100B in myoblasts regulates the transition from activation to quiescence and from quiescence to activation, and reduces apoptosis. Biochim Biophys Acta. 2011;1813:1092–104. doi: 10.1016/j.bbamcr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 111.Arcuri C, Bianchi R, Brozzi F, Donato R. S100B increases proliferation in PC12 neuronal cells and reduces their responsiveness to NGF via Akt activation. J Biol Chem. 2005;280:4402–14. doi: 10.1074/jbc.M406440200. [DOI] [PubMed] [Google Scholar]
- 112.Donato R. Mechanism of action of S-100 protein(s) on the assembly of brain microtubule proteins. Biochem Biophys Res Commun. 1984;124:850–6. doi: 10.1016/0006-291x(84)91035-0. [DOI] [PubMed] [Google Scholar]
- 113.Garbuglia M, Verzini M, Donato R. Annexin VI binds S100A1 and S100B and blocks the ability of S100A1 and S100B to inhibit desmin and GFAP assemblies into intermediate filaments. Cell Calcium. 1998;24:177–91. doi: 10.1016/s0143-4160(98)90127-0. [DOI] [PubMed] [Google Scholar]
- 114.Garbuglia M, Verzini M, Rustandi RR, et al. Role of the C-terminal extension in the interaction of S100A1 with GFAP, tubulin, the S100A1- and S100B-inhibitory peptide, TRTK-12, and a peptide derived from p53, and the S100A1 inhibitory effect on GFAP polymerization. Biochem Biophys Res Commun. 1998;254:36–41. doi: 10.1006/bbrc.1998.9881. [DOI] [PubMed] [Google Scholar]
- 115.Garbuglia M, Verzini M, Hofmann A, Huber R, Donato R. S100A1 and S100B interactions with annexins. Biochim Biophys Acta. 2000;1498:192–206. doi: 10.1016/s0167-4889(00)00096-3. [DOI] [PubMed] [Google Scholar]
- 116.Donato R. Calcium-independent, pH-regulated effects of S-100 proteins on assembly-disassembly of brain microtubule protein in vitro. J Biol Chem. 1998;263:106–10. [PubMed] [Google Scholar]
- 117.Bianchi R, Giambanco I, Donato R. S-100 protein, but not calmodulin, binds to and inhibits the polymerization of the glial fibrillary acidic protein in a Ca2+-dependent manner. J Biol Chem. 1993;268:12669–74. [PubMed] [Google Scholar]
- 118.Garbuglia M, Verzini M, Giambanco I, Spreca A, Donato R. Effects of calcium-binding proteins (S100a0, S100a, S100b) on desmin assembly in vitro. FASEB J. 1996;10:317–24. doi: 10.1096/fasebj.10.2.8641565. [DOI] [PubMed] [Google Scholar]
- 119.Sorci G, Agneletti AL, Donato R. Effects of S100A1 and S100B on microtubule stability. An in vitro study using tritoncytoskeletons from astrocyte and myoblast cell lines. Neuroscience. 2000;99:773–83. doi: 10.1016/s0306-4522(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 120.van Dieck J, Teufel DP, Jaulent AM, et al. Posttranslational modifications affect the interaction of S100 proteins with tumor suppressor p53. J Mol Biol. 2009;394:922–30. doi: 10.1016/j.jmb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Gilquin B, Cannon BR, Hubstenberger A, et al. The calcium-dependent interaction between S100B and the mitochondrial AAA ATPase ATAD3A and the role of this complex in the cytoplasmic processing of ATAD3A. Mol Cell Biol. 2010;30:2724–36. doi: 10.1128/MCB.01468-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiong Z, O'Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A. Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res. 2000;257:281–9. doi: 10.1006/excr.2000.4902. [DOI] [PubMed] [Google Scholar]
- 123.Gentil BJ, Delphin C, Mbele GO, et al. The giant protein AHNAK is a specific target for the calcium- and zinc-binding S100B protein: potential implications for Ca2+ homeostasis regulation by S100B. J Biol Chem. 2001;276:23253–61. doi: 10.1074/jbc.M010655200. [DOI] [PubMed] [Google Scholar]
- 124.Tsoporis JN, Overgaard CB, Izhar S, Parker TG. S100B modulates the hemodynamic response to norepinephrine stimulation. Am J Hypertens. 2009;22:1048–53. doi: 10.1038/ajh.2009.145. [DOI] [PubMed] [Google Scholar]
- 125.Sahoo N, Tröger J, Heinemann SH, Schönherr R. Current inhibition of human EAG1 potassium channels by the Ca2+ binding protein S100B. FEBS Lett. 2010;584:3896–900. doi: 10.1016/j.febslet.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 126.Liu J, Wang H, Zhang L, et al. S100B transgenic mice develop features of Parkinson's disease. Arch Med Res. 2011;42:1–7. doi: 10.1016/j.arcmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y, Buck DC, Neve KA. Novel interaction of the dopamine D2 receptor and the Ca2+-binding protein S100B: role in D2 receptor function. Mol Pharmacol. 2008;74:371–8. doi: 10.1124/mol.108.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rambotti MG, Giambanco I, Spreca A, Donato R. S100B and S100A1 proteins in bovine retina: their calcium-dependent stimulation of a membrane-bound guanylate cyclase activity as investigated by ultracytochemistry. Neuroscience. 1999;92:1089–101. doi: 10.1016/s0306-4522(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 129.Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol. 2003;457:404–19. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- 130.Schroeter ML, Abdul-Khaliq H, Sache J, Steiner J, Blasig IE, Mueller K. Mood disorders are glial disorders: evidence from in vivo studies. Cardiovasc Psychiat Neurol. 2010;2010:780645. doi: 10.1155/2010/780645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rothermundt M, Ahn JN, Jörgens S. S100B in schizophrenia: an update. Gen Physiol Biophys. 2009;28:F76–81. [PubMed] [Google Scholar]
- 132.McIlroy M, McCartan D, Early S, et al. Interaction of developmental transcription factor HOXC11 with steroid receptor coactivator SRC-1 mediates resistance to endocrine therapy in breast cancer [corrected]. Cancer Res. 2010;70:1585–94. doi: 10.1158/0008-5472.CAN-09-3713. [DOI] [PubMed] [Google Scholar]
- 133.Kleindienst A, Schmidt C, Parsch H, Emtmann I, Xu Y, Buchfelder M. The passage of S100B from brain to blood is not specifically related to the blood-brain barrier integrity. Cardiovasc Psychiat Neurol. 2010;2010:801295. doi: 10.1155/2010/801295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vos PE, Jacobs B, Andriessen TM, et al. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–93. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 135.Luu KC, Nie GY, Salamonsen LA. Endometrial calbindins are critical for embryo implantation: evidence from in vivo use of morpholino antisense oligonucleotides. Proc Natl Acad Sci USA. 2004;101:8028–33. doi: 10.1073/pnas.0401069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malmendal A, Vander Kooi CW, Nielsen NC, Chazin WJ. Calcium-modulated S100 protein-phospholipid interactions. An NMR study of calbindin D9k and DPC. Biochemistry. 2005;44:6502–12. doi: 10.1021/bi050088z. [DOI] [PubMed] [Google Scholar]
- 137.Austermann J, Nazmi AR, Müller-Tidow C, Gerke V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem. 2008;283:29331–40. doi: 10.1074/jbc.M806145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du M, Wang G, Ismail TM, et al. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem. 2012;287:15330–44. doi: 10.1074/jbc.M112.349787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heil A, Nazmi AR, Koltzscher M, et al. S100P is a novel interaction partner and regulator of IQGAP1. J Biol Chem. 2011;286:7227–38. doi: 10.1074/jbc.M110.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gribenko AV, Hopper JE, Makhatadze GI. Molecular characterization and tissue distribution of a novel member of the S100 family of EF-hand proteins. Biochemistry. 2001;40:15538–48. doi: 10.1021/bi0114731. [DOI] [PubMed] [Google Scholar]
- 141.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6:121–38. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 142.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 143.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. Nat Clin Pract Rheumatol. 2007;3:382–90. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 144.Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85:1373–80. doi: 10.1002/jnr.21211. [DOI] [PubMed] [Google Scholar]
- 145.Dassan P, Keir G, Brown MM. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis. 2009;27:295–302. doi: 10.1159/000199468. [DOI] [PubMed] [Google Scholar]
- 146.Gogas H, Eggermont AM, Hauschild A, et al. Biomarkers in melanoma. Ann Oncol. 2009;20(Suppl 6):vi8–13. doi: 10.1093/annonc/mdp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gazzolo D, Michetti F. Perinatal S100B protein assessment in human unconventional biological fluids: A minireview and new perspectives. Cardiovasc Psychiatry Neurol. 2010;2010:703563. doi: 10.1155/2010/703563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tramontina AC, Tramontina F, Bobermin LD, et al. Secretion of S100B, an astrocyte-derived neurotrophic protein, is stimulated by fluoxetine via a mechanism independent of serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1580–83. doi: 10.1016/j.pnpbp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 149.de Souza DF, Leite MC, Quincozes-Santos A, et al. S100B secretion is stimulated by IL-1β in glial cultures and hippocampal slices of rats: Likely involvement of MAPK pathway. J Neuroimmunol. 2009;206:52–7. doi: 10.1016/j.jneuroim.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Nardin P, Tortorelli L, Quincozes-Santos A, et al. S100B secretion in acute brain slices: Modulation by extracellular levels of Ca2+ and K+. Neurochem Res. 2009;34:1603–11. doi: 10.1007/s11064-009-9949-0. [DOI] [PubMed] [Google Scholar]
- 151.Lawrie A, Spiekerkoetter E, Martinez EC, et al. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–35. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- 152.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–41. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 153.Steiner J, Marquardt N, Pauls I, et al. Human CD8+ T cells and NK cells express and secrete S100B upon stimulation. Brain Behav Immun. 2011;25:1233–41. doi: 10.1016/j.bbi.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 154.Van Eldik LJ, Zimmer DB. Secretion of S-100 from rat C6 glioma cells. Brain Res. 1987;436:367–70. doi: 10.1016/0006-8993(87)91681-7. [DOI] [PubMed] [Google Scholar]
- 155.Steiner J, Bernstein HG, Bogerts B, et al. S100B is expressed in, and released from, OLN-93 oligodendrocytes: Influence of serum and glucose deprivation. Neuroscience. 2008;154:496–503. doi: 10.1016/j.neuroscience.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 156.Netto CB, Conte S, Leite MC, et al. Serum S100B protein is increased in fasting rats. Arch Med Res. 2006;37:683–6. doi: 10.1016/j.arcmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Steiner J, Walter M, Guest PC, et al. Elevated S100B levels in schizophrenia are associated with insulin resistance. Mol Psychiatry. 2010;15:3–4. doi: 10.1038/mp.2009.87. [DOI] [PubMed] [Google Scholar]
- 158.Kathir KM, Ibrahim K, Rajalingam D, Prudovsky I, Yu C, Kumar TK. S100A13-lipid interactions-role in the non-classical release of the acidic fibroblast growth factor. Biochim Biophys Acta. 2007;1768:3080–9. doi: 10.1016/j.bbamem.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kapoor M, Zhou Q, Otero F, et al. Evidence for annexin II S100A10 complex and plasmin in mobilization of cytokine activity of human TrpRS. J Biol Chem. 2008;283:2070–7. doi: 10.1074/jbc.M706028200. [DOI] [PubMed] [Google Scholar]
- 160.Curatola G, Ferretti G, Mazzanti L, Donato R. S-100 protein-induced changes in the physical state of synaptosomal particulate fractions as monitored by spin labels. Arch Biochem Biophys. 1985;240:435–45. doi: 10.1016/0003-9861(85)90048-7. [DOI] [PubMed] [Google Scholar]
- 161.Zolese G, Tangorra A, Curatola G, Giambanco I, Donato R. Interaction of S-100b protein with cardiolipin vesicles as monitored by electron spin resonance, pyrene fluorescence and circular dichroism. Cell Calcium. 1988;9:149–57. doi: 10.1016/0143-4160(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 162.Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fritz G, Botelho HM, Morozova-Roche LA, Gomes CM. Natural and amyloid self-assembly of S100 proteins: structural basis of functional diversity. FEBS J. 2010;277:4578–90. doi: 10.1111/j.1742-4658.2010.07887.x. [DOI] [PubMed] [Google Scholar]
- 164.Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009;86:577–87. doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- 165.Lim SY, Raftery MJ, Geczy CL. Oxidative Modifications of DAMPs Suppress Inflammation: The Case for S100A8 and S100A9. Antioxid Redox Signal. 2011;15:2235–48. doi: 10.1089/ars.2010.3641. [DOI] [PubMed] [Google Scholar]
- 166.Ma W, Lee SE, Guo J, et al. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48:1355–61. doi: 10.1167/iovs.06-0738. [DOI] [PubMed] [Google Scholar]
- 167.Hernandez-Ochoa EO, Prosser BL, Wright NT, Contreras M, Weber DJ, Schneider MF. Augmentation of Cav1 channel current and action potential duration after uptake of S100A1 in sympathetic ganglion neurons. Am J Physiol Cell Physiol. 2009;297:C955–70. doi: 10.1152/ajpcell.00140.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rezvanpour A, Shaw GS. Unique S100 target protein interactions. Gen Physiol Biophys. 2009;28:F39–46. Spec No Focus. [PubMed] [Google Scholar]
- 169.Yan WX, Armishaw C, Goyette J, et al. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J Biol Chem. 2008;283:13035–43. doi: 10.1074/jbc.M710388200. [DOI] [PubMed] [Google Scholar]
- 170.Ostendorp T, Leclerc E, Galichet A, et al. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007;26:3868–78. doi: 10.1038/sj.emboj.7601805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leclerc E, Fritz G, Weibel M, Heizmann CW, Galichet A. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J Biol Chem. 2007;282:31317–31. doi: 10.1074/jbc.M703951200. [DOI] [PubMed] [Google Scholar]
- 172.Xie J, Burz DS, He W, Bronstein IB, Lednev I, Shekhtman A. Hexameric calgranulin C (S100A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J Biol Chem. 2007;82:4218–31. doi: 10.1074/jbc.M608888200. [DOI] [PubMed] [Google Scholar]
- 173.Moroz OV, Burkitt W, Wittkowski H, et al. Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC Biochem. 2009;10:11. doi: 10.1186/1471-2091-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hermani A, De Servi B, Medunjaninm S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-κB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–97. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 175.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–48. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bjork P, Björk A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 178.Sparvero LJ, Asafu-Adjei D, Kang R, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 180.Srikrishna G, Nayak J, Weigle B, et al. Carboxylated N-glycans on RAGE promote S100A12 binding and signaling. J Cell Biochem. 2010;110:645–59. doi: 10.1002/jcb.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids. 2009;36:381–9. doi: 10.1007/s00726-008-0097-7. [DOI] [PubMed] [Google Scholar]
- 182.Hoppmann S, Steinbach J, Pietzsch J. Scavenger receptors are associated with cellular interactions of S100A12 in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:651–61. doi: 10.1016/j.biocel.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 183.Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40:241–8. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- 184.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277:3658–65. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- 185.Kiryushko D, Novitskaya V, Soroka V, et al. Molecular mechanisms of Ca2+ signaling in neurons induced by the S100A4 protein. Mol Cell Biol. 2006;26:3625–38. doi: 10.1128/MCB.26.9.3625-3638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reppel M, Sasse P, Piekorz R, et al. S100A1 enhances the L-type Ca2+ current in embryonic mouse and neonatal rat ventricular cardiomyocytes. J Biol Chem. 2005;280:36019–28. doi: 10.1074/jbc.M504750200. [DOI] [PubMed] [Google Scholar]
- 187.Komada T, Araki R, Nakatani K, Yada I, Naka M, Tanaka T. Novel specific chemotactic receptor for S100L protein on guinea pig eosinophils. Biochem Biophys Res Commun. 1996;220:871–4. doi: 10.1006/bbrc.1996.0496. [DOI] [PubMed] [Google Scholar]
- 188.Balmain N, Moutahir F, Heizmann CW, Lieberherr M. Immunolocalization of S100A2 calcium-binding protein in cartilage and bone cells. Cell Mol Biol (Noisy-le-grand) 2003;49:485–6. [PubMed] [Google Scholar]
- 189.Schmidt-Hansen B, Ornås D, Grigorian M, et al. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene. 2004;23:5487–95. doi: 10.1038/sj.onc.1207720. [DOI] [PubMed] [Google Scholar]
- 190.Semov A, Moreno MJ, Onichtchenko A, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–41. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 191.Grum-Schwensen B, Klingelhöfer J, Grigorian M, et al. Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a T-cell deficiency in primary tumors. Cancer Res. 2010;70:936–47. doi: 10.1158/0008-5472.CAN-09-3220. [DOI] [PubMed] [Google Scholar]
- 192.Takenaga K, Nakamura Y, Sakiyama S. Expression of a calcium binding protein pEL98 (mts1) during differentiation of human promyelocytic leukemia HL-60 cells. Biochem Biophys Res Commun. 1994;202:94–101. doi: 10.1006/bbrc.1994.1898. [DOI] [PubMed] [Google Scholar]
- 193.Forst B, Hansen MT, Klingelhöfer J, et al. Metastasis-inducing S100A4 and RANTES cooperate in promoting tumor progression in mice. PLoS ONE. 2010;5:e10374. doi: 10.1371/journal.pone.0010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Klingelhöfer J, Møller HD, Sumer EU, et al. Epidermal growth factor receptor ligands as new extracellular targets for the metastasis-promoting S100A4 protein. FEBS J 009. 276:5936–48. doi: 10.1111/j.1742-4658.2009.07274.x. [DOI] [PubMed] [Google Scholar]
- 195.Kondo N, Ichimiya S, Tamura Y, et al. A calcium binding protein, S100A4, mediates T cell dependent cytotoxicity as a transformation-associated antigen. Microbiol Immunol. 2005;49:49–56. doi: 10.1111/j.1348-0421.2005.tb03639.x. [DOI] [PubMed] [Google Scholar]
- 196.Kato C, Kojima T, Komaki M, et al. S100A4 inhibition by RNAi up-regulates osteoblast related genes in periodontal ligament cells. Biochem Biophys Res Commun. 2005;326:147–53. doi: 10.1016/j.bbrc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 197.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 198.Yammani RR, Long D, Loeser RF. Interleukin-7 stimulates secretion of S100A4 by activating the JAK/STAT signaling pathway in human articular chondrocytes. Arthritis Rheum. 2009;60:792–800. doi: 10.1002/art.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schneider M, Kostin S, Strøm CC, et al. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res. 2007;75:40–50. doi: 10.1016/j.cardiores.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 200.Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med. 2008;86:507–22. doi: 10.1007/s00109-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 201.Gläser R, Meyer-Hoffert U, Harder J, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol. 2009;129:641–9. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- 202.Abtin A, Eckhart L, Mildner M, Gruber F, Schröder JM, Tschachler E. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 2008;22:2168–76. doi: 10.1096/fj.07-104117. [DOI] [PubMed] [Google Scholar]
- 203.Gläser R, Harder J, Lange H, Bartels J, Christophers E, Schröder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 204.Lee KC, Eckert RL. S100A7 (Psoriasin)--mechanism of antibacterial action in wounds. J Invest Dermatol. 2007;127:945–57. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- 205.Wolf R, Howard OM, Dong HF, et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. 2008;181:1499–506. doi: 10.4049/jimmunol.181.2.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng Y, Niyonsaba F, Ushio H, et al. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology. 2008;124:357–67. doi: 10.1111/j.1365-2567.2007.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qin W, Ho L, Wang J, Peskind E, Pasinetti GM. S100A7, a novel Alzheimer's disease biomarker with non-amyloidogenic alpha-secretase activity acts via selective promotion of ADAM-10. PLoS One. 2009;4:e4183. doi: 10.1371/journal.pone.0004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lackmann M, Rajasekariah P, Iismaa SE, et al. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP-10. J Immunol. 1993;50:2981–91. [PubMed] [Google Scholar]
- 209.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 210.Devery J, King N, Geczy C. Acute inflammatory activity of the S100 protein CP-10 activation of neutrophil in vivo and in vitro. J Immunol. 1994;152:1888–97. [PubMed] [Google Scholar]
- 211.Cornish CJ, Devery JM, Poronnik P, Lackmann M, Cook DI, Geczy C. S100 protein CP-10 stimulates myeloid cell chemotaxis without activation. J Cell Physiol. 1996;166:427–437. doi: 10.1002/(SICI)1097-4652(199602)166:2<427::AID-JCP21>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 212.Nishimura F, Terranova VP, Sawa T, Murayama Y. Migration inhibitory factor related protein-8 (MRP-8) is an autocrine chemotactic factor for periodontal ligament cells. J Dent Res. 1999;78:1251–5. doi: 10.1177/00220345990780060901. [DOI] [PubMed] [Google Scholar]
- 213.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–35. [PubMed] [Google Scholar]
- 214.van Lent PL, Grevers LC, Schelbergen R, et al. S100A8 causes a shift toward expression of activatory Fc receptors on macrophages via toll-like receptor 4 and regulates Fc receptor expression in synovium during chronic experimental arthritis. Arthritis Rheum. 2010;62:3353–64. doi: 10.1002/art.27654. [DOI] [PubMed] [Google Scholar]
- 215.van Lent PL, Grevers LC, Blom AB, et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 2008;58:3776–87. doi: 10.1002/art.24074. [DOI] [PubMed] [Google Scholar]
- 216.Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–92. doi: 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hsu K, Passey RJ, Endoh Y, et al. Regulation of S100A8 by glucocorticoids. J Immunol. 2005;174:2318–26. doi: 10.4049/jimmunol.174.4.2318. [DOI] [PubMed] [Google Scholar]
- 218.Endoh Y, Chung YM, Clark IA, Geczy CL, Hsu K. IL-10-dependent S100A8 gene induction in monocytes/macrophages by double-stranded RNA. J Immunol. 2009;182:2258–68. doi: 10.4049/jimmunol.0802683. [DOI] [PubMed] [Google Scholar]
- 219.Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 triggers oxidation-sensitive repulsion of neutrophils. J Dent Res. 2006;85:829–33. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McCormick MM, Rahimi F, Bobryshev YV, et al. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–9. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 221.Zhao J, Endoh I, Hsu K, Tedla N, Endoh Y, Geczy CL. S100a8 modulates mast cell function and suppresses eosinophil migration in acute asthma. Antioxid Redox Signal. 2011;14:1589–600. doi: 10.1089/ars.2010.3583. [DOI] [PubMed] [Google Scholar]
- 222.Caldwell RL, Opalenik SR, Davidson JM, Caprioli RM, Nanney LB. Tissue profiling MALDI mass spectrometry reveals prominent calcium-binding proteins in the proteome of regenerative MRL mouse wounds. Wound Repair Regen. 2008;16:442–9. doi: 10.1111/j.1524-475X.2007.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sroussi HY, Williams RL, Zhang QL, Villines D, Marucha PT. Ala42S100A8 ameliorates psychological-stress impaired cutaneous wound healing. Brain Behav Immun. 2009;23:755–9. doi: 10.1016/j.bbi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schnekenburger J, Schick V, Krüger B, et al. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell-cell contacts. J Cell Physiol. 2008;216:558–67. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- 225.Anceriz N, Vandal K, Tessier PA. S100A9 mediates neutrophil adhesion to fibronectin through activation of β2 integrins. Biochem Biophys Res Commun. 2007;354:84–9. doi: 10.1016/j.bbrc.2006.12.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Simard JC, Girard D, Tessier PA. Induction of neutrophil degranulation by S100A9 via a MAPK-dependent mechanism. J Leukoc Biol. 2010;87:905–14. doi: 10.1189/jlb.1009676. [DOI] [PubMed] [Google Scholar]
- 227.Simard JC, Simon MM, Tessier PA, Girard D. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol. 2011;186:3622–31. doi: 10.4049/jimmunol.1002956. [DOI] [PubMed] [Google Scholar]
- 228.Sunahori K, Yamamura M, Yamana J, et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aguiar-Passeti T, Postol E, Sorg C, Mariano M. Epithelioid cells from foreign-body granuloma selectively express the calcium-binding protein MRP-14, a novel down-regulatory molecule of macrophage activation. J Leukoc Biol. 1997;62:852–8. doi: 10.1002/jlb.62.6.852. [DOI] [PubMed] [Google Scholar]
- 230.Shibata F, Miyama K, Shinoda F, et al. Fibroblast growth-stimulating activity of S100A9 (MRP-14). Eur J Biochem. 2004;271:2137–43. doi: 10.1111/j.1432-1033.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 231.Ahmad A, Bayley DL, He S, Stockley RA. Myeloid related protein-8/14 stimulates interleukin-8 production in airway epithelial cells. Am J Respir Cell Mol Biol. 2003;29:523–30. doi: 10.1165/rcmb.2002-0286OC. [DOI] [PubMed] [Google Scholar]
- 232.Thurgood LA, Wang T, Chataway TK, Ryall RL. Comparison of the specific incorporation of intracrystalline proteins into urinary calcium oxalate monohydrate and dihydrate crystals. J Proteome Res. 2010;9:4745–57. doi: 10.1021/pr100467z. [DOI] [PubMed] [Google Scholar]
- 233.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–24. doi: 10.1189/jlb.0706433. [DOI] [PubMed] [Google Scholar]
- 234.Pagano RL, Sampaio SC, Juliano MA, Juliano L, Giorgi R. Involvement of proteinase-activated receptors 1 and 2 in spreading and phagocytosis by murine adherent peritoneal cells: modulation by the C-terminal of S100A9 protein. Eur J Pharmacol. 2010;628:240–6. doi: 10.1016/j.ejphar.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 235.De Lorenzo BH, Godoy LC, Novaes e Brito RR, et al. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2010;215:341–7. doi: 10.1016/j.imbio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 236.Yin LM, Li HY, Zhang QH, et al. Effects of S100A9 in a rat model of asthma and in isolated tracheal spirals. Biochem Biophys Res Commun. 2010;398:547–52. doi: 10.1016/j.bbrc.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 237.Shimizu K, Libby P, Rocha VZ, et al. Loss of myeloid related protein-8/14 exacerbates cardiac allograft rejection. Circulation. 2011;124:2920–32. doi: 10.1161/CIRCULATIONAHA.110.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dale CS, Altier C, Cenac N, et al. Analgesic properties of S100A9 C-terminal domain: a mechanism dependent on calcium channel inhibition. Fundam Clin Pharmacol. 2009;23:427–38. doi: 10.1111/j.1472-8206.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 239.Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting -helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370:887–98. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 240.Kehl-Fie TE, Chitayat S, Hood MI, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–64. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Corbin BD, Seeley EH, Raab A, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–5. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 242.Pechkovsky DV, Zalutskaya OM, Ivanov GI, Misuno NI. Calprotectin (MRP8/14 protein complex) release during mycobacterial infection in vitro and in vivo. FEMS Immunol Med Microbiol. 2000;29:27–33. doi: 10.1111/j.1574-695X.2000.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 243.Ryckman C, Robichaud GA, Roy J, Cantin R, Tremblay MJ, Tessier PA. HIV-1 transcription and virus production are both accentuated by the proinflammatory myeloid-related proteins in human CD4+ T lymphocytes. J Immunol. 2002;169:3307–13. doi: 10.4049/jimmunol.169.6.3307. [DOI] [PubMed] [Google Scholar]
- 244.Yanamandra K, Alexeyev O, Zamotin V, et al. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLoS ONE. 2009;4:e5562. doi: 10.1371/journal.pone.0005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sinha P, Okoro C, Foell D, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 247.Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem. 2010;285:10822–31. doi: 10.1074/jbc.M109.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gebhardt C, Riehl A, Durchdewald M, Németh J, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–85. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ghavami S, Rashedi I, Dattilo BM, et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83:1484–92. doi: 10.1189/jlb.0607397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–43. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pouliot P, Plante I, Raquil MA, Tessier PA, Olivier M. Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response. J Immunol. 2008;181:3595–601. doi: 10.4049/jimmunol.181.5.3595. [DOI] [PubMed] [Google Scholar]
- 252.Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007;376:197–204. doi: 10.1016/j.cca.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 253.Sroussi HY, Lu Y, Zhang QL, Villines D, Marucha PT. S100A8 and S100A9 inhibit neutrophil oxidative metabolism in-vitro: involvement of adenosine metabolites. Free Radic Res. 2010;44:389–96. doi: 10.3109/10715760903431434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Loser K, Vogl T, Voskort M, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713–7. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 255.Brun JG, Ulvestad E, Fagerhol MK, Jonsson R. Effects of human calprotectin (L1) on in vitro immunoglobulin synthesis. Scand J Immunol. 1994;40:75–680. doi: 10.1111/j.1365-3083.1994.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 256.Nukui T, Ehama R, Sakaguchi M, et al. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem. 2008;104:453–64. doi: 10.1002/jcb.21639. [DOI] [PubMed] [Google Scholar]
- 257.Ehlermann P, Eggers K, Bierhaus A, et al. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rector CL, Murphy RC. Determination of leukotriene A(4) stabilization by S100A8/A9 proteins using mass spectrometry. J Lipid Res. 2009;50:2064–71. doi: 10.1194/jlr.M900017-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Viemann D, Barczyk K, Vogl T, et al. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109:2453–60. doi: 10.1182/blood-2006-08-040444. [DOI] [PubMed] [Google Scholar]
- 260.Seeliger S, Vogl T, Engels IH, et al. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol. 2003;163:947–56. doi: 10.1016/S0002-9440(10)63454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–60. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]
- 262.Ghavami S, Chitayat S, Hashemi M, et al. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625:73–83. doi: 10.1016/j.ejphar.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 263.Ghavami S, Eshragi M, Ande SR, et al. S100A8/A9 induces autophagy and apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes that involves BNIP3. Cell Res. 2010;20:314–31. doi: 10.1038/cr.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yui S, Nakatani Y, Hunter MJ, Chazin WJ, Yamazaki M. Implication of extracellular zinc exclusion by recombinant human calprotectin (MRP8 and MRP14) from target cells in its apoptosis-inducing activity. Mediators Inflamm. 2002;11:165–72. doi: 10.1080/09622935020138208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Manitz MP, Horst B, Seeliger S, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–43. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Källberg E, Vogl T, Liberg D, et al. S100A9 interaction with TLR4 promotes tumor growth. PLoS ONE. 2012;7:e34207. doi: 10.1371/journal.pone.0034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Farris SD, Hu JH, Krishnan R, et al. Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: Role of the uPA receptor and S100A8/A9 proteins. J Biol Chem. 2011;286:22665–77. doi: 10.1074/jbc.M110.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–46. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 269.O'Connell PA, Madureira PA, Berman JN, Liwski RS, Waisman DM. Regulation of S100A10 by the PML-RAR- oncoprotein. Blood. 2011;117:4095–105. doi: 10.1182/blood-2010-07-298851. [DOI] [PubMed] [Google Scholar]
- 270.Kwon M, MacLeod TJ, Zhang Y, Waisman DM. S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front Biosci. 2005;10:300–25. doi: 10.2741/1529. [DOI] [PubMed] [Google Scholar]
- 271.Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118:3172–81. doi: 10.1182/blood-2011-05-353482. [DOI] [PubMed] [Google Scholar]
- 272.O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136–46. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- 273.Phipps KD, Surette AP, O'Connell PA, Waisman DM. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Cancer Res. 2011;71:6676–83. doi: 10.1158/0008-5472.CAN-11-1748. [DOI] [PubMed] [Google Scholar]
- 274.Yang X, Popescu NC, Zimonjic DB. DLC1 interaction with S100A10 mediates inhibition of in vitro cell invasion and tumorigenicity of lung cancer cells through a RhoGAP-independent mechanism. Cancer Res. 2011;71:2916–25. doi: 10.1158/0008-5472.CAN-10-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Celma CC, Roy P. Interaction of calpactin light chain (S100A10/p11) and a viral NS protein is essential for intracellular trafficking of nonenveloped bluetongue virus. J Virol. 2011;85:4783–91. doi: 10.1128/JVI.02352-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hanaue M, Miwa N, Uebi T, Fukuda Y, Katagiri Y, Takamatsu K. Characterization of S100A11, a suppressive factor of fertilization, in the mouse female reproductive tract. Mol Reprod Dev. 2010;78:91–103. doi: 10.1002/mrd.21273. [DOI] [PubMed] [Google Scholar]
- 277.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol 2055. 175:8296–302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 278.Cecil DL, Terkeltaub R. Transamidation by transglutaminase 2 transforms S100A11 calgranulin into a procatabolic cytokine for chondrocytes. J Immunol. 2008;180:8378–85. doi: 10.4049/jimmunol.180.12.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Fuellen G, Nacken W, Sorg C, Kerkhoff C. Computational searches for missing orthologs: the case of S100A12 in mice. OMICS. 2004;8:334–40. doi: 10.1089/omi.2004.8.334. [DOI] [PubMed] [Google Scholar]
- 280.Ravasi T, Hsu K, Goyette J, et al. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84:10–22. doi: 10.1016/j.ygeno.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 281.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69:986–94. [PubMed] [Google Scholar]
- 282.Moroz OV, Blagova EV, Wilkinson AJ, Wilson KS, Bronstein IB. The crystal structures of human S100A12 in apo form and in complex with zinc: new insights into S100A12 oligomerisation. J Mol Biol. 2009;391:536–51. doi: 10.1016/j.jmb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 283.Cole AM, Kim YH, Tahk S, et al. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001;504:5–10. doi: 10.1016/s0014-5793(01)02731-4. [DOI] [PubMed] [Google Scholar]
- 284.Yang Z, Yan WX, Cai H, et al. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119:106–14. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 285.Goyette J, Yan WX, Yamen E, et al. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183:593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]
- 286.Rouleau P, Vandal K, Ryckman C, et al. The calcium-binding protein S100A12 induces neutrophil adhesion, migration, and release from bone marrow in mouse at concentrations similar to those found in human inflammatory arthritis. Clin Immunol. 2003;107:46–54. doi: 10.1016/s1521-6616(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 287.Mikkelsen SE, Novitskaya V, Kriajevska M, et al. S100A12 protein is a strong inducer of neurite outgrowth from primary hippocampal neurons. J Neurochem. 2001;79:767–76. doi: 10.1046/j.1471-4159.2001.00605.x. [DOI] [PubMed] [Google Scholar]
- 288.Moroz OV, Antson AA, Grist SJ, et al. Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr. 2003;59:859–67. doi: 10.1107/s0907444903004700. [DOI] [PubMed] [Google Scholar]
- 289.Hsieh HL, Schäfer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun. 2004;316:949–59. doi: 10.1016/j.bbrc.2004.02.135. [DOI] [PubMed] [Google Scholar]
- 290.Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol. 2005;67:87–101. doi: 10.1016/j.jri.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 291.Jin Q, Chen H, Luo A, Ding F, Liu Z. S100A14 Stimulates Cell Proliferation and Induces Cell Apoptosis at Different Concentrations via Receptor for Advanced Glycation End Products (RAGE). PLoS ONE. 2011;6:e19375. doi: 10.1371/journal.pone.0019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wolf R, Lewerenz V, Büchau AS, Walz M, Ruzicka T. Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp Dermatol. 2007;16:685–91. doi: 10.1111/j.1600-0625.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 293.Büchau AS, Hassan M, Kukova G, et al. S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. J Invest Dermatol. 2007;127:2596–604. doi: 10.1038/sj.jid.5700946. [DOI] [PubMed] [Google Scholar]
- 294.Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- 295.Sorci G, Bianchi R, Riuzzi F, et al. S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovas Psychiatry Neurol. 2010;pii:656481. doi: 10.1155/2010/656481. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through RAGE activation. J Biol Chem. 2000;275:40096–105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 297.Businaro R, Leone S, Fabrizi C, et al. S100B protects neurons against Aβ amyloid-induced neurotoxicity via RAGE engagement at low doses and increases Aβ amyloid neurotoxicity at high doses. J Neurosci Res. 2006;83:897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- 298.Reali C, Scintu F, Pillai R, Donato R, Michetti F, Sogos V. S100B counteracts effects of the neurotoxicant trimethyltin on astrocytes and microglia. J Neurosci Res. 2005;81:677–86. doi: 10.1002/jnr.20584. [DOI] [PubMed] [Google Scholar]
- 299.Zhang L, Liu W, Alizadeh D, et al. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–98. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Petrova V, Hu J, Van Eldik LJ. Modulation of glial activation by astrocyte-derived protein S100B: differential responses of astrocyte and microglial cultures. Brain Res. 2000;853:74–80. doi: 10.1016/s0006-8993(99)02251-9. [DOI] [PubMed] [Google Scholar]
- 301.Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia. 2001;33:131–42. [PubMed] [Google Scholar]
- 302.Adami C, Bianchi R, Pula G, Donato R. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta. 2004;1742:169–77. doi: 10.1016/j.bbamcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 303.Bianchi R, Adami C, Giambanco I, Donato R. The S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol. 2007;81:108–18. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- 304.Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-κB and AP-1. Co-regulation of COX-2 expression by S100B and IL-1β and TNF-α. Neurobiol Aging. 2010;31:665–77. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 305.Bianchi R, Kastrisianaki E, Giambanco I, Donato R. S100B protein stimulates microglia migration via RAGE-dependent upregulation of chemokine expression and release. J Biol Chem. 2011;286:7214–26. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mori T, Tan J, Arendash GW, Koyama N, Nojima Y, Town T. Overexpression of human S100B exacerbates brain damage and periinfarct gliosis after permanent focal ischemia. Stroke. 2008;39:2114–21. doi: 10.1161/STROKEAHA.107.503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wainwright MS, Craft JM, Griffin WS, et al. Increased susceptibility of S100B transgenic mice to perinatal hypoxiaischemia. Ann Neurol. 2004;56:61–7. doi: 10.1002/ana.20142. [DOI] [PubMed] [Google Scholar]
- 308.Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 2010;58:300–14. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–55. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- 310.Kleindienst A, Bullock MR. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 311.Willoughby KA, Kleindienst A, Müller C, Chen T, Muir JK, Ellis EF. S100B protein is released by in vitro trauma and reduces delayed neuronal injury. J Neurochem. 2004;91:1284–91. doi: 10.1111/j.1471-4159.2004.02812.x. [DOI] [PubMed] [Google Scholar]
- 312.Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101:1463–70. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- 313.Yan SS, Wu ZY, Zhang HP, et al. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med. 2003;9:287–93. doi: 10.1038/nm831. [DOI] [PubMed] [Google Scholar]
- 314.Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci USA. 2002;99:4037–42. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Shanmugam N, Reddy MA, Natarajan R. Distinct roles of heterogeneous nuclear ribonuclear protein K and microRNA-16 in cyclooxygenase-2 RNA stability induced by S100b, a ligand of the receptor for advanced glycation end products. J Biol Chem. 2008;283:36221–33. doi: 10.1074/jbc.M806322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. FASEB J. 2006;20:1695–7. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- 317.Muramatsu Y, Kurosaki R, Watanabe H, et al. Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia. 2003;42:307–13. doi: 10.1002/glia.10225. [DOI] [PubMed] [Google Scholar]
- 318.Iuvone T, Esposito G, De Filippis D, et al. Cannabinoid CB1 receptor stimulation affords neuroprotection in MPTP-induced neurotoxicity by attenuating S100B up-regulation in vitro. J Mol Med. 2007;85:1379–92. doi: 10.1007/s00109-007-0233-y. [DOI] [PubMed] [Google Scholar]
- 319.Choudhury ME, Moritoyo T, Kubo M, et al. Zonisamide-induced long-lasting recovery of dopaminergic neurons from MPTP-toxicity. Brain Res. 2011;1384:170–8. doi: 10.1016/j.brainres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 320.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2011;108:949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 322.Hearst SM, Walker LR, Shao Q, Lopez M, Raucher D, Vig PJ. The design and delivery of a thermally responsive peptide to inhibit S100B-mediated neurodegeneration. Neuroscience. 2011;197:369–80. doi: 10.1016/j.neuroscience.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sbai O, Devi TS, Melone MA, et al. RAGE-TXNIP axis is required for S100B-promoted Schwann cell migration, fibronectin expression and cytokine secretion. J Cell Sci. 2010;123:4332–9. doi: 10.1242/jcs.074674. [DOI] [PubMed] [Google Scholar]
- 324.Rong LL, Trojaborg W, Qu W, et al. Antagonism of RAGE suppresses peripheral nerve regeneration. FASEB J. 2004;18:1812–17. doi: 10.1096/fj.04-1899com. [DOI] [PubMed] [Google Scholar]
- 325.Rong LL, Yan SF, Wendt T, et al. RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. FASEB J. 2004;18:1818–25. doi: 10.1096/fj.04-1900com. [DOI] [PubMed] [Google Scholar]
- 326.Sorci G, Riuzzi F, Agneletti AL, Marchetti C, Donato R. S100B inhibits myogenic differentiation and myotube formation in a RAGE-independent manner. Mol Cell Biol. 2003;23:4870–81. doi: 10.1128/MCB.23.14.4870-4881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Riuzzi F, Sorci G, Donato R. S100B stimulates myoblast proliferation and inhibits myoblast differentiation by independently stimulating ERK1/2 and inhibiting p38 MAPK. J Cell Physiol. 2006;207:461–70. doi: 10.1002/jcp.20580. [DOI] [PubMed] [Google Scholar]
- 328.Riuzzi F, Sorci G, Beccafico S, Donato R. S100B engages RAGE or bFGF/FGFR1 in myoblasts depending on its own concentration and myoblast density. Implications for muscle regeneration. PLoS ONE. 2001;7:e28700. doi: 10.1371/journal.pone.0028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–44. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 330.Chen Y, Yan SS, Colgan J, et al. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173:1399–405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 331.Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia. 2006;49:100–7. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- 332.Reddy MA, Li SL, Sahar S, et al. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–93. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 333.Curran CS, Bertics PJ. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. Int Immunol. 2011;23:713–28. doi: 10.1093/intimm/dxr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mu L, Zhang Y, Sun B, et al. Activation of the receptor for advanced glycation end products (RAGE) exacerbates experimental autoimmune myasthenia gravis symptoms. Clin Immunol. 2011;141:36–48. doi: 10.1016/j.clim.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 335.Tsoporis JN, Mohammadzadeh F, Parker TG. Intracellular and extracellular effects of S100B in the cardiovascular response to disease. Cardiovasc Psychiatry Neurol. 2010;2010:206073. doi: 10.1155/2010/206073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 337.Tsoporis JN, Izhar S, Proteau G, Slaughter G, Parker TG. S100B-RAGE dependent VEGF secretion by cardiac myocytes induces myofibroblast proliferation. J Mol Cell Cardiol. 2011;52:464–73. doi: 10.1016/j.yjmcc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 338.Shaw SS, Schmidt AM, Banes AK, Wang X, Stern DM, Marrero MB. S100B-RAGE-mediated augmentation of angiotensin II-induced activation of JAK2 in vascular smooth muscle cells is dependent on PLD2. Diabetes. 2003;52:2381–8. doi: 10.2337/diabetes.52.9.2381. [DOI] [PubMed] [Google Scholar]
- 339.Arumugam T, Logsdon CD. S100P: a novel therapeutic target for cancer. Amino Acids. 2011;41:893–9. doi: 10.1007/s00726-010-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37–51. doi: 10.1016/j.cccn.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 341.Manolakis AC, Kapsoritakis AN, Tiaka EK, Potamianos SP. Calprotectin, calgranulin C, and other members of the s100 protein family in inflammatory bowel disease. Dig Dis Sci. 2011;56:1601–11. doi: 10.1007/s10620-010-1494-9. [DOI] [PubMed] [Google Scholar]
- 342.Sampson B, Fagerhol MK, Sunderkotter C, et al. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002;360:1742–5. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- 343.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–31. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 344.Judd TA, Day AS, Lemberg DA, Turner D, Leach ST. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol. 2011;26:1493–9. doi: 10.1111/j.1440-1746.2011.06846.x. [DOI] [PubMed] [Google Scholar]
- 345.Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32:223–9. doi: 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Schaub N, Reichlin T, Meune C, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–56. doi: 10.1373/clinchem.2011.172940. [DOI] [PubMed] [Google Scholar]
- 347.Morrow DA, Wang Y, Croce K, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Baumann M, Schmaderer C, Burkhardt K, et al. MRP8/14 is associated with systemic inflammation in stable coronary atherosclerosis in men. Eur J Clin Invest. 2011;41:1261–7. doi: 10.1111/j.1365-2362.2011.02530.x. [DOI] [PubMed] [Google Scholar]
- 349.Andrés Cerezo L, Mann H, Pecha O, et al. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther. 2011;13:R122. doi: 10.1186/ar3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Baillet A, Trocmé C, Berthier S, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology (Oxford) 2010;49:671–82. doi: 10.1093/rheumatology/kep452. [DOI] [PubMed] [Google Scholar]
- 351.Hammer HB, Ødegård S, Syversen SW, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:150–4. doi: 10.1136/ard.2008.103739. [DOI] [PubMed] [Google Scholar]
- 352.Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Kikkawa T, Sato N, Kojika M, et al. Significance of measuring S100A12 and sRAGE in the serum of sepsis patients with postoperative acute lung injury. Dig Surg. 2010;27:307–12. doi: 10.1159/000313687. [DOI] [PubMed] [Google Scholar]
- 354.Takahashi G, Hoshikawa K, Matsumoto N, et al. Changes in serum S100A12 and sRAGE associated with improvement of the PaO2/FiO2 ratio following PMX-DHP therapy for postoperative septic shock. Eur Surg Res. 2011;47:135–40. doi: 10.1159/000330448. [DOI] [PubMed] [Google Scholar]
- 355.Zhao F, Hoechst B, Duffy A, et al. S100A9 a new marker for monocytic human myeloid derived suppressor cells. Immunology. 2012;136:176–83. doi: 10.1111/j.1365-2567.2012.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kawai H, Minamiya Y, Takahashi N. Prognostic impact of S100A9 overexpression in non-small cell lung cancer. Tumour Biol. 2011;32:641–6. doi: 10.1007/s13277-011-0163-8. [DOI] [PubMed] [Google Scholar]
- 357.Xiao MB, Jiang F, Ni WK, et al. High expression of S100A11 in pancreatic adenocarcinoma is an unfavorable prognostic marker. Med Oncol. 2012;29(3):1886–91. doi: 10.1007/s12032-011-0058-y. [DOI] [PubMed] [Google Scholar]
- 358.Stein U, Burock S, Herrmann P, et al. Diagnostic and prognostic value of metastasis inducer S100A4 transcripts in plasma of colon, rectal, and gastric cancer patients. J Mol Diagn. 2011;13:189–98. doi: 10.1016/j.jmoldx.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Tripathi SC, Matta A, Kaur J, et al. Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One. 2010;5:e11939. doi: 10.1371/journal.pone.0011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Michetti F, Corvino V, Geloso MC, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–59. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- 361.Gazzolo D, Lituania M, Bruschettini M, et al. S100B protein levels in saliva: correlation with gestational age in normal term and preterm newborns. Clin Biochem. 2005;38:229–33. doi: 10.1016/j.clinbiochem.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 362.Michetti F, Bruschettini M, Frigiola A, et al. Saliva S100B in professional sportsmen: High levels at resting conditions and increased after vigorous physical activity. Clin Biochem. 2011;44:245–7. doi: 10.1016/j.clinbiochem.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 363.Leite MC, Galland F, Brolese G, et al. A simple, sensitive and widely applicable ELISA for S100B: Methodological features of the measurement of this glial protein. J Neurosci Methods. 2008;169:93–9. doi: 10.1016/j.jneumeth.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 364.Gazzolo D, Florio P, Zullino E, et al. S100B protein increases in human blood and urine during stressful activity. Clin Chem Lab Med. 2010;48:1363–5. doi: 10.1515/CCLM.2010.262. [DOI] [PubMed] [Google Scholar]
- 365.Gazzolo D, Bruschettini M, Corvino V, et al. S100b protein concentrations in amniotic fluid correlate with gestational age and with cerebral ultrasound scanning results in healthy fetuses. Clin Chem. 2001;47:954–6. [PubMed] [Google Scholar]
- 366.Gazzolo D, Monego G, Corvino V, et al. Human milk contains S100B protein. Biochim Biophys Acta. 2003;1619:209–12. doi: 10.1016/s0304-4165(02)00499-3. [DOI] [PubMed] [Google Scholar]
- 367.Fassbender K, Schmidt R, Schreiner A, et al. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci. 1997;148:101–5. doi: 10.1016/s0022-510x(96)05351-8. [DOI] [PubMed] [Google Scholar]
- 368.Buttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961–5. doi: 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- 369.Weiss N, Sanchez-Pena P, Roche S, et al. Prognosis value of plasma S100B protein levels after subarachnoid aneurysmal hemorrhage. Anesthesiology. 2006;104:658–66. doi: 10.1097/00000542-200604000-00008. Erratum in: Anesthesiology 2008; 108: 767.
- 370.Nylen K, Ost M, Csajbok LZ, et al. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien) 2008;150:221–7. doi: 10.1007/s00701-007-1489-2. discussion 227. [DOI] [PubMed] [Google Scholar]
- 371.Anderson RE, Hansson LO, Nilsson O, Liska J, Settergren G, Vaage J. Increase in serum S100A1-B and S100BB during cardiac surgery arises from extracerebral sources. Ann Thorac Surg. 2001;71:1512–7. doi: 10.1016/s0003-4975(01)02399-2. [DOI] [PubMed] [Google Scholar]
- 372.Pelinka LE, Bahrami S, Szalay L, Umar F, Redl H. Hemorrhagic shock induces an S 100B increase associated with shock severity. Shock. 2003;19:422–6. doi: 10.1097/01.shk.0000055345.58165.52. [DOI] [PubMed] [Google Scholar]
- 373.Routsi C, Stamataki E, Nanas S, et al. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26:20–4. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- 374.Mazzini GS, Schaf DV, Vinade ER, et al. Increased S100B serum levels in dilated cardiomyopathy patients. J Card Fail. 2007;13:850–4. doi: 10.1016/j.cardfail.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 375.Mazzini GS, Schaf DV, Oliveira AR, et al. The ischemic rat heart releases S100B. Life Sci. 2005;77:882–9. doi: 10.1016/j.lfs.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 376.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–8. doi: 10.1097/00006123-200106000-00012. discussion 1258-1260. [DOI] [PubMed] [Google Scholar]
- 377.Unden J, Christensson B, Bellner J, Alling C, Romner B. Scand J Infect Dis. 2004;36:10–3. doi: 10.1080/00365540310017294. [DOI] [PubMed] [Google Scholar]
- 378.Pelinka L, Szalay L, Jafarmadar M, Schmidhammer R, Redl H, Bahrami S. Circulating S100B is increased after bilateral femur fracture without brain injury in the rat. Br J Anaesthesia. 2003;91:595–7. doi: 10.1093/bja/aeg225. [DOI] [PubMed] [Google Scholar]
- 379.Wiesmann M, Wandinger KP, Missler U, et al. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol Psychiatry. 1999;45:1508–11. doi: 10.1016/s0006-3223(98)00217-0. [DOI] [PubMed] [Google Scholar]
- 380.Lara DR, Gama CS, Belmonte-de-Abreu P, et al. Increased serum S100B protein in schizophrenia: a study in medication-free patients. J Psychiatr Res. 2001;35:11–4. doi: 10.1016/s0022-3956(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 381.Rothermundt M, Arolt V, Wiesmann M, et al. S-100B is increased in melancholic but not in non-melancholic major depression. J Affect Disord. 2011;66:89–91. doi: 10.1016/s0165-0327(00)00321-9. [DOI] [PubMed] [Google Scholar]
- 382.Machado-Vieira R, Lara DR, Portela LV, et al. Elevated serum S100B protein in drug-free bipolar patients during first manic episode: a pilot study. Eur Neuropsychopharmacol. 2002;12:269–72. doi: 10.1016/s0924-977x(02)00029-9. [DOI] [PubMed] [Google Scholar]
- 383.Steiner J, Schiltz K, Walter M, et al. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology. 2010;35:321–4. doi: 10.1016/j.psyneuen.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 384.Michetti F, Massaro A, Murazio M. The nervous system-specific S-100 antigen in cerebrospinal fluid of multiple sclerosis patients. Neurosci Lett. 1979;11:171–5. doi: 10.1016/0304-3940(79)90122-8. [DOI] [PubMed] [Google Scholar]
- 385.Gazzolo D, Grutzfeld D, Michetti F, et al. Increased S100B in cerebrospinal fluid of infants with bacterial meningitis: relationship to brain damage and routine cerebrospinal fluid findings. Clin Chem. 2004;50:941–4. doi: 10.1373/clinchem.2003.021048. [DOI] [PubMed] [Google Scholar]
- 386.Sindic CJ, Freund M, Van Regemorter N, Verellen-Dumoulin C, Masson PL. S-100 protein in amniotic fluid of anencephalic fetuses. Prenat Diagn. 1984;4:297–302. doi: 10.1002/pd.1970040409. [DOI] [PubMed] [Google Scholar]
- 387.Gazzolo D, Bruschettini M, Corvino V, et al. Amniotic fluid levels of S100B protein in normal and trisomy-21 foetuses. Clin Chim Acta. 2003;330:131–3. doi: 10.1016/s0009-8981(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 388.Tort AB, Portela LV, da Purificação Tavares M, et al. Specificity and sensitivity of S100B levels in amniotic fluid for Down syndrome diagnosis. Life Sci. 2004;76:379–84. doi: 10.1016/j.lfs.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 389.Bhattacharya S, Bunick CG, Chazin WJ. Target selectivity in EF-hand calcium binding proteins. Biochim Biophys Acta. 2004;1742:69–79. doi: 10.1016/j.bbamcr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 390.Ostendorp T, Diez J, Heizmann CW, Fritz G. The crystal structures of human S100B in the zinc- and calcium-loaded state at three pH values reveal zinc ligand swapping. Biochim Biophys Acta. 2011;1813:1083–91. doi: 10.1016/j.bbamcr.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 391.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–14. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Weber DJ, Rustandi R, Carrier F, Zimmer DB. In: CALCIUM: The Molecular Basis of Calcium Action in Biology and Medicine. Pochet R, editor. Kluwer Academic Publishers; Dordrecht: 2000. pp. 521–39. [Google Scholar]
- 393.Markowitz J, Chen I, Gitti R, et al. Identification and characterization of small molecule inhibitors of the calcium-dependent S100B-p53 tumor suppressor interaction. J Med Chem. 2004;47:5085–93. doi: 10.1021/jm0497038. [DOI] [PubMed] [Google Scholar]
- 394.Markowitz J, Mackerell AD, Jr, Carrier F, Charpentier TH, Weber DJ. Design of inhibitors for S100B. Curr Top Med Chem. 2005;5:1093–108. doi: 10.2174/156802605774370865. [DOI] [PubMed] [Google Scholar]
- 395.Charpentier TH, Wilder PT, Liriano MA, et al. Small molecules bound to unique sites in the target protein binding cleft of calcium-bound S100B as characterized by nuclear magnetic resonance and X-ray crystallography. Biochemistry. 2009;48:6202–12. doi: 10.1021/bi9005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wright NT, Cannon BR, Zimmer DB, Weber DJ. S100A1: Structure, Function, and Therapeutic Potential. Curr Chem Biol. 2009;3:138–45. doi: 10.2174/187231309788166460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wilder PT, Lin J, Bair CL, et al. Recognition of the tumor suppressor protein p53 and other protein targets by the calcium-binding protein S100B. Biochim Biophys Acta. 2006;1763:1284–97. doi: 10.1016/j.bbamcr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 398.Wilder PT, Charpentier TH, Liriano M, et al. In vitro screening and structural characterization of inhibitors of the S100B-p53 interaction. Int J High-Throughput Screening. 2010;(1):109–126. doi: 10.2147/IJHTS.S8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Shuker SB, Hajduk PJ, Meadows RP, Fesik SW. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274:1531–4. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 400.Huang Q, Petros AM, Virgin HW, Fesik SW, Olejniczak ET. Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc Natl Acad Sci USA. 2002;99:3428–33. doi: 10.1073/pnas.062525799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Leclerc E, Heizmann CW. The importance of Ca2+/Zn2+ signaling S100 proteins and RAGE in translational medicine. Front Biosci (Schol Ed) 2011;3:1232–62. doi: 10.2741/223. [DOI] [PubMed] [Google Scholar]
- 402.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Reddy TR, Li C, Guo X, Myrvang HK, Fischer PM, Dekker LV. Design, synthesis, and structure-activity relationship exploration of 1-substituted 4-aroyl-3-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one analogues as inhibitors of the annexin A2-S100A10 protein interaction. J Med Chem. 2011;54:2080–94. doi: 10.1021/jm101212e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Garrett SC, Hodgson L, Rybin A, et al. A biosensor of S100A4 metastasis factor activation: inhibitor screening and cellular activation dynamics. Biochemistry. 2008;47:986–96. doi: 10.1021/bi7021624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.House RP, Pozzuto M, Patel P, et al. Two functional S100A4 monomers are necessary for regulating nonmuscle myosin-IIA and HCT116 cell invasion. Biochemistry. 2011;50:6920–32. doi: 10.1021/bi200498q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Mack GS, Marshall A. Lost in migration. Nat Biotechnol. 2010;28:214–29. doi: 10.1038/nbt0310-214. [DOI] [PubMed] [Google Scholar]
- 407.Elenjord R, Ljones H, Sundkvist E, Loennechen T, Winberg JO. Dysregulation of matrix metalloproteinases and their tissue inhibitors by S100A4. Connect Tissue Res. 2008;49:185–8. doi: 10.1080/03008200802143125. [DOI] [PubMed] [Google Scholar]
- 408.Markowitz J, MacKerell AD, Jr., Weber DJ. A search for inhibitors of S100B, a member of the S100 family of calcium-binding proteins. Mini Rev Med Chem. 2007;7:609–16. doi: 10.2174/138955707780859422. [DOI] [PubMed] [Google Scholar]
- 409.Donato R. Chlorpromazine binding to S-100 protein. J Neurochem. 1984;42:1468–71. doi: 10.1111/j.1471-4159.1984.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 410.Donato R. Chlorpromazine inhibits the calcium-mediated effects of S-100 protein(s) on assembled brain microtubule proteins, but not those on microtubule protein assembly. Biochem Biophys Res Commun. 1984;122:983–90. doi: 10.1016/0006-291x(84)91188-4. [DOI] [PubMed] [Google Scholar]
- 411.Lin J, Yang Q, Yan Z, et al. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. J Biol Chem. 2004;279:34071–7. doi: 10.1074/jbc.M405419200. [DOI] [PubMed] [Google Scholar]
- 412.Matsumoto T, Murao S, Kito K, Kihana T, Matsuura S, Ueda N. Modulation of S-100 genes response to growth conditions in human epithelial tumor cells. Pathol Int. 1997;47:339–46. doi: 10.1111/j.1440-1827.1997.tb04506.x. [DOI] [PubMed] [Google Scholar]
- 413.Sack U, Walther W, Scudiero D, et al. S100A4-induced cell motility and metastasis is restricted by the Wnt/ -catenin pathway inhibitor calcimycin in colon cancer cells. Mol Biol Cell. 2011;22:3344–54. doi: 10.1091/mbc.E10-09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sack U, Walther W, Scudiero D, et al. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J Natl Cancer Inst. 2011;103:1018–36. doi: 10.1093/jnci/djr190. [DOI] [PubMed] [Google Scholar]
- 415.deBlacam C, Byrne C, Hughes E, et al. HOXC11-SRC-1 regulation of S100beta in cutaneous melanoma: new targets for the kinase inhibitor dasatinib. Br J Cancer. 2011;105:118–23. doi: 10.1038/bjc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Stoll SW, Zhao X, Elder JT. EGF stimulates transcription of CaN19 (S100A2) in HaCaT keratinocytes. J Invest Dermatol. 1998;111:1092–7. doi: 10.1046/j.1523-1747.1998.00402.x. [DOI] [PubMed] [Google Scholar]
- 417.Leite MC, Galland F, de Souza DF, et al. Gap junction inhibitors modulate S100B secretion in astrocyte cultures and acute hippocampal slices. J Neurosci Res. 2009;87:2439–46. doi: 10.1002/jnr.22083. [DOI] [PubMed] [Google Scholar]
- 418.Hu J, Castets F, Guevara JL, Van Eldik LJ. S100 stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J Biol Chem. 1996;271:2543–7. doi: 10.1074/jbc.271.5.2543. [DOI] [PubMed] [Google Scholar]
- 419.Hu J, Van Eldik LJ. S100 induces apoptotic cell death in cultured astrocytes via a nitric oxide-dependent pathway. Biochim Biophys Acta. 1996;1313:239–45. doi: 10.1016/0167-4889(96)00095-x. [DOI] [PubMed] [Google Scholar]
- 420.Lam AG, Koppal T, Akama KT, et al. Mechanism of glial activation by S100B: involvement of the transcription factor NFκ B. Neurobiol Aging. 2001;22:765–72. doi: 10.1016/s0197-4580(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 421.Hu J, Ferreira A, Van Eldik LJ. S100β induces neuronal cell death through nitric oxide release from astrocytes. J Neurochem. 1997;69:2294–301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- 422.Watanabe Y, Kato H, Araki T. Protective action of neuronal nitric oxide synthase inhibitor in the MPTP mouse model of Parkinson's disease. Metab Brain Dis. 2008;23:51–69. doi: 10.1007/s11011-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 423.Rani SG, Mohan SK, Yu C. Molecular level interactions of S100A13 with amlexanox: inhibitor for formation of the multiprotein complex in the nonclassical pathway of acidic fibroblast growth factor. Biochemistry. 2010;49:2585–92. doi: 10.1021/bi9019077. [DOI] [PubMed] [Google Scholar]
- 424.Lee K, Yun ST, Yun CO, Ahn BY, Jo EC. S100A2 promoter-driven conditionally replicative adenovirus targets non-small-cell lung carcinoma. Gene Ther. 2011 doi: 10.1038/gt.2011.168. doi: 10.1038/gt.2011.168. [DOI] [PubMed] [Google Scholar]
- 425.Most P, Remppis A, Pleger ST, Katus HA, Koch WJ. S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R568–77. doi: 10.1152/ajpregu.00075.2007. [DOI] [PubMed] [Google Scholar]