Figure 6.
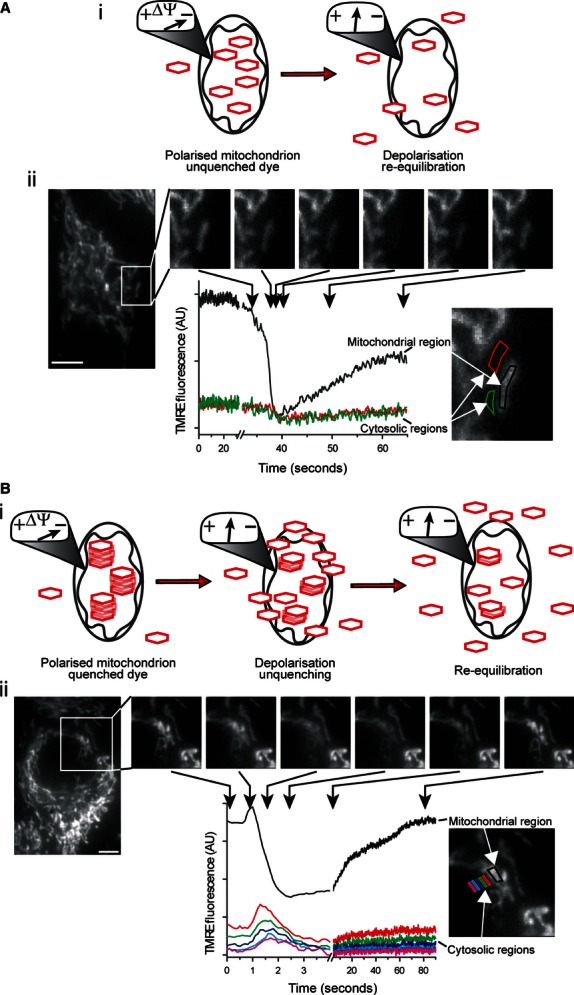
Membrane-permeant, lipophilic fluorescent cations can be used to monitor ΔΨM in “quenched” and “unquenched” modes. These fluorophores equilibrate across membranes in a Nernstian fashion (i.e., ∼10-fold per 60 mV). (A) at low fluorophore concentration the observed fluorescence is proportional to ΔΨM (i). A transient ΔΨM depolarization of an individual mitochondrion in a primary cerebral vascular smooth muscle cell loaded with TMRE (100 nM) resulted in a transient decrease in the fluorescence intensity of the mitochondrial region (ii, black line) with no measureable fluorescence change in the surrounding cytosolic regions (ii, red & green lines) (ii, scale bar = 5 μm), as visualized by epifluorescence microscopy; that is the change in [TMRE] outside the mitochondria, after ΔΨM depolarization, is presumably rapidly decreased in the larger volume of the cytoplasm so that a change in fluorescence is not measured. (B) At higher concentrations these fluorophores aggregate in mitochondria and self-quench, so that the observed fluorescence is no longer directly proportional to ΔΨM. ΔΨM depolarization relieves this quenching, resulting in a transient increase in fluorescence (Bi & Bii). In this case, a transient ΔΨM depolarization of an individual mitochondrion resulted in a transient increase in the fluorescence intensity of the mitochondrial region (Bii, black line) and an observable dissipation of the released fluorophore into the surrounding cytosolic regions (Bii, colored lines) (ii, scale bar = 5 μm) because of the larger amount of fluorophore being released from mitochondria to the cytoplasm. When the dye is fully unquenched, the fluorescent signal declines as the dye continues to leave the mitochondria.
