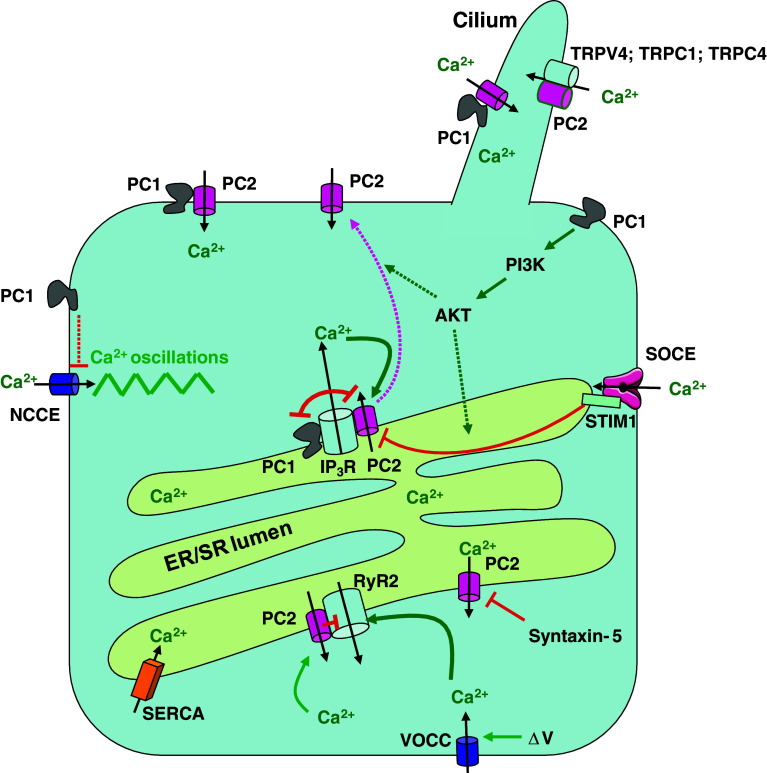
Fig. 1.
Major players controlling cellular Ca2+ signaling by polycystins. Polycystin-1 (PC1) and polycystin-2 (PC2) form a signaling complex in the cilium that mediates Ca2+ influx via PC2, possibly in response to mechanical stimuli. Also TRPV4, TRPC1, and TRPC4 interact with PC2 and could play a role in mechano-sensitive Ca2+ influx. PC2 is also present in the ER where it directly interacts with the IP3R and in cardiac cells also with the RyR2. PC2 behaves as a Ca2+-induced Ca2+-release channel and thereby amplifies IP3-induced Ca2+ release. The RyR2 is activated by Ca2+ influx via voltage-operated Ca2+ channels and is inhibited by PC2. Ca2+ leak via PC2 may be controlled by other proteins such as syntaxin-5. PC1 activates the PI3-K/AKT signaling. This leads (by as-yet-unresolved mechanisms) to an increase in the STIM1-IP3R interaction, which reduces the interaction between the IP3R and PC2 with possibly a translocation of PC2 to the plasma membrane. PC1 and PC2 compete for the same binding site on the IP3R. PC1 dysfunction leads to strengthening of the IP3R-PC2 interaction and remodeling of the Ca2+ fluxes with an increase of IICR, more ER Ca2+ depletion, and Ca2+ influx via activation of SOCE. PC1 also negatively modulates agonist-evoked NCCE activity through a still undefined mechanism. Loss of function of PC1 causes an increase in NCCE-channel activity leading to Ca2+ oscillations. PC1/PC2 polycystin-1/-2, NCCE non-capacitive Ca2+ entry, ΔV voltage change over the plasma membrane, VOCC voltage-operated Ca2+ channel. Inhibitory and stimulatory mechanisms are represented by red and green arrows, respectively; the purple arrow represents the trafficking of PC2; dotted lines indicate that the mechanisms are as yet undefined