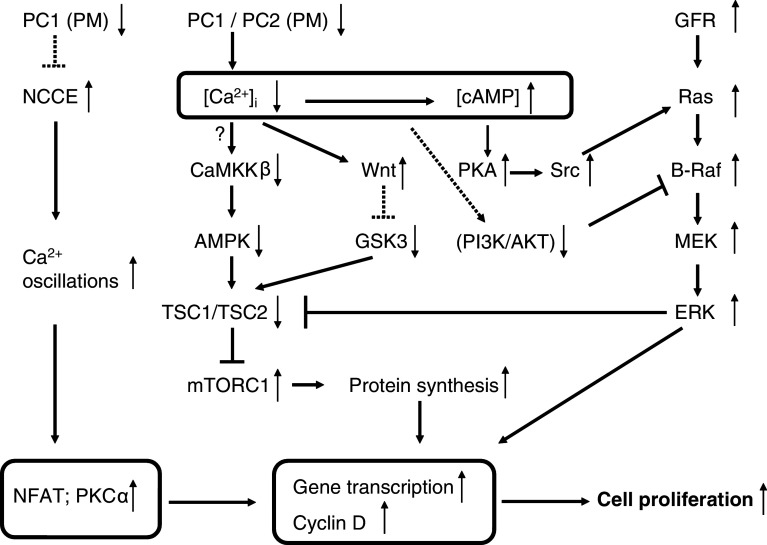
Fig. 2.
Signaling pathways that relate disturbed polycystin-mediated Ca2+ signaling to cell proliferation. Defects in polycystin functions generally lead to a decrease in the cytosolic Ca2+ concentration ([Ca2+]i). This results in an increase in cAMP concentration, probably via changes in Ca2+-dependent phosphodiesterase or Ca2+-dependent adenylate-cyclase activity (not shown). The Ca2+-cAMP link is represented by the box. In the presence of low [Ca2+]i, cAMP becomes pro-proliferative via activation of the Src/Ras/B-Raf/MEK/ERK pathway. Ca2+ restriction causes decreased PI3-K/AKT signaling, which relieves the inhibition of B-Raf. Activation of growth-factor receptors with tyrosine-kinase activity (GFR) also contributes to the stimulation of MAPK/ERK signaling and cell proliferation. Polycystin-1 dysfunction thus upregulates the MAPK/ERK pathway, which results in inactivation of the tuberin complex and increased mTORC1. Cytoplasmic Ca2+ also regulates the mTOR pathway via CaMKKß and AMPK (the question mark indicates that the occurrence of this mechanism was not yet explored in cystic cells). The deregulated Ca2+ signaling switches on canonical Wnt signaling, which activates mTOR via inhibiting GSK3 phosphorylation of tuberin. Another link to cell proliferation may depend on the activation of Ca2+ oscillations and subsequent effects on gene transcription and cyclins via Ca2+-dependent NFAT or via protein kinase Cα (PKCα) signaling. Boxed areas indicate mechanisms not shown in detail. Dotted arrows indicate still-unresolved mechanisms. GFR growth-factor receptor