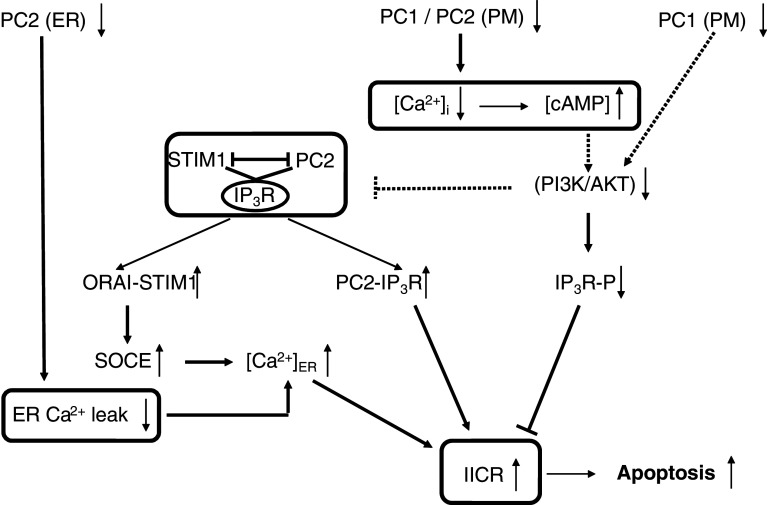
Fig. 3.
Signaling pathways relating disturbed polycystin-mediated Ca2+ signaling to apoptotic cell death. Disruption of PC1 function leads to a phenotype with low intracellular Ca2+ concentration and high cAMP concentration that showed a mitogenic response towards cAMP and down-regulation of PI3-K/AKT. This provokes a profound remodeling of the relation between Ca2+ release and Ca2+ influx via an IP3R/PC2/STIM1 protein complex. Decreased AKT signaling would strengthen the IP3R-PC2 interaction and lead to increased IICR, translocation of STIM1 to the plasma membrane, and refilling of the ER via SOCE. AKT can also directly phosphorylate the IP3R thereby inhibiting its activity. A decreased AKT activity in cystic cells would thereby relieve the inhibition of the IP3R and contribute to the increase in IICR. PC2 can function as an ER Ca2+-leak channel and a loss of function would therefore increase the ER Ca2+ content and IICR. The increased IICR via these different mechanisms ultimately leads to increased Ca2+ transfer from the ER to the mitochondria. Mitochondrial Ca2+ overload is a very important determinant of Ca2+-dependent apoptosis. Boxed areas indicate mechanisms not shown in detail. Dotted arrows indicate still unresolved mechanisms. IP3R-P: IP3R phosphorylated by AKT (S2681 in IP3R1)