Abstract
During the course of human immunodeficiency virus type 1 (HIV-1) disease, the virus has been shown to effectively escape the immune response with the subsequent establishment of latent viral reservoirs in specific cell populations within the peripheral blood (PB) and associated lymphoid tissues, bone marrow (BM), brain, and potentially other end organs. HIV-1, along with hepatitis B and C viruses (HBV and HCV), are known to share similar routes of transmission, including intravenous drug use, blood transfusions, sexual intercourse, and perinatal exposure. Substance abuse, including the use of opioids and cocaine, is a significant risk factor for exposure to HIV-1 and the development of acquired immune deficiency syndrome, as well as HBV and HCV exposure, infection, and disease. Thus, coinfection with HIV-1 and HBV or HCV is common and may be impacted by chronic substance abuse during the course of disease. HIV-1 impacts the natural course of HBV and HCV infection by accelerating the progression of HBV/HCV-associated liver disease toward end-stage cirrhosis and quantitative depletion of the CD4+ T-cell compartment. HBV or HCV coinfection with HIV-1 is also associated with increased mortality when compared to either infection alone. This review focuses on the impact of substance abuse and coinfection with HBV and HCV in the PB, BM, and brain on the HIV-1 pathogenic process as it relates to viral pathogenesis, disease progression, and the associated immune response during the course of this complex interplay. The impact of HIV-1 and substance abuse on hepatitis virus-induced disease is also a focal point.
Keywords: bone marrow, brain, cocaine, HBV, HCV, HIV-1, opioids
INTRODUCTION
HIV Disease and Its Relationship with Coinfection and Substance Abuse
According to the 2010 UNAIDS report, approximately 1.5 million people in the United States are infected with the human immunodeficiency virus type 1 (HIV-1), whereas globally, an estimated 33.3 million people are living with HIV infection or the acquired immune deficiency syndrome (AIDS) [1]. During initial acute infection with HIV-1, the resultant innate immune response and the eventual adaptive immune response ultimately curb the primary infection, leading to the establishment of a basal viral load or “viral set point.” After acute infection, the patient undergoes a period of clinical latency that involves intermittent bursts of viral replication in the regional lymph nodes with small amounts of virus periodically being shed into the peripheral blood (PB) compartment and elsewhere [2]. During the acute phase of HIV-1 infection, the virus is likely seeded into a number of tissues and associated cellular reservoirs that may include the brain [3], lung, gastrointestinal tract [4], kidney [5], genital tract [6], bone marrow (BM) [7], and other tissues, with at least one of the cellular reservoirs including cells of the monocyte-macrophage lineage [8] or resting memory CD4+ T cells [9] present within the PB compartment and regional lymph nodes [10]. Although a number of therapeutic strategies have resulted in some success regarding the long-term control of HIV-1 infection and disease progression, eradicating latent virus from these cellular reservoirs has proven to be a major obstacle not yet overcome by antiretroviral therapy strategies used to date. Nevertheless, these reservoirs can be considered reasonable and important targets for therapeutic intervention [11, 12]. In addition to the cells of the monocyte-macrophage lineage, progenitor cell populations within the BM (Fig. 1A) have also been shown to harbor HIV-1 proviral DNA (Fig. 1B). This infected cellular reservoir may be a critical factor in the etiology of disease within the central nervous system (CNS) and perhaps other end organs [13-15]. The BM consists of many different cell types, one of them being CD34+ hematopoietic progenitor cells (HPCs) (Fig. 1A). Certain subpopulations of HPCs express CD4, CCR5, and/or CXCR4, the HIV-1 receptor and coreceptors, respectively [13, 16]. An in vivo study has shown that CD34+ HPCs were infected with HIV-1 in a subset of seropositive individuals [17]. Because the progenitor cell population has a substantial proliferative capacity, these cells could generate infected cell lineages that can disseminate the infection to the brain and other end organs [13, 15]. The BM is also the site of hematopoiesis, and HIV-1-infected patients are often diagnosed with a wide variety of hematologic abnormalities [13]. Thus, the BM likely plays a pivotal role in HIV-1 pathogenesis and disease.
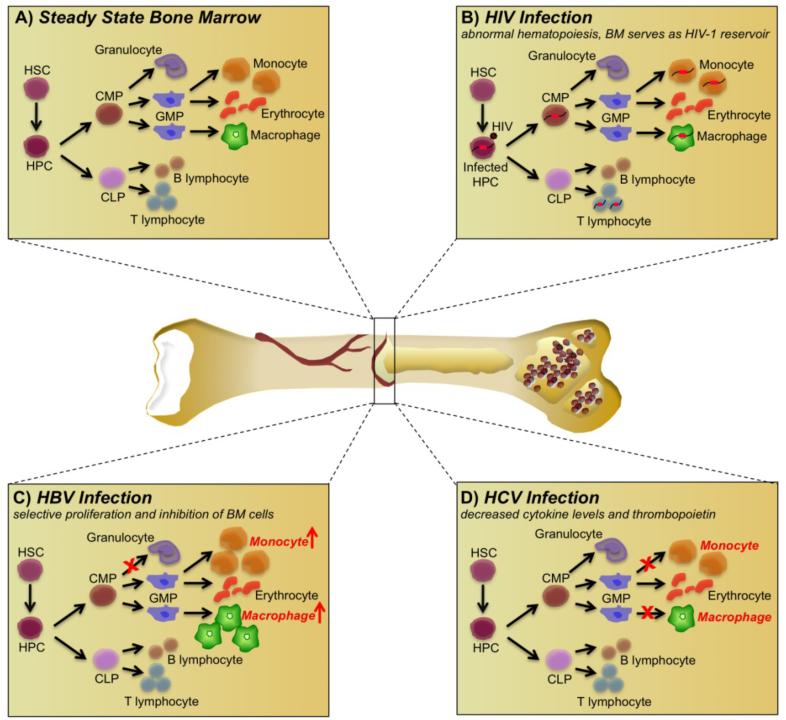
Fig. (1).
Effects of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) on bone marrow (BM) biology. (A) The steady state within the BM compartment consists of hematopoietic stem cells (HSC), which can differentiate into hematopoietic progenitor cells (HPC). The HPCs can further differentiate into various committed progenitor populations such as committed lymphoid progenitors (CLP) and committed myeloid progenitors (CMP). CMPs can further differentiate into granuloctye-macrophage progenitors (GMPs), which develop into monocytes and erythrocytes. The CLPs can develop into B and T lymphocytes. In addition, dendritic cells, fibroblasts, and mesenchymal stem cells form the BM microenvironment. (B) HIV exerts a global effect on the BM, infecting HPCs, myeloid cells, and stromal cells, leading to an alteration in cytokine profiles within the BM, abnormal hematopoiesis, and growth patterns, and forming a latent reservoir in the HPCs. (C) HBV exerts a selective effect on BM hematopoiesis, wherein progenitor cells committed to the monocyte-macrophage lineage are induced into differentiation; the resultant monocytes and macrophages are induced into proliferating, while progenitors committed to differentiating into granulocytes are inhibited from proliferating. (D) HCV exerts an inhibitory effect on the monocyte-macrophage progenitors, thus hampering the proliferation of monocytes, macrophages, and erythrocytes, resulting in anemia in patients with chronic HCV, accompanied by a decrease in thrombopoietin and cytokine levels, responsible for platelet production.
Hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV-1 are known to share similar routes of transmission and can be transmitted via parenteral intravenous drug use (IVDU) and blood transfusions and sexual and perinatal exposure. Thus, coinfection with HIV-1 and HBV and/or HCV is commonly encountered [18, 19]. In fact, about 2 billion people worldwide have been infected with HBV, a DNA virus; of that number, 350 million remain chronically infected, which is the cause of approximately 30% of all cases of liver cirrhosis and 53% of the cases of hepatocellular carcinoma (HCC) [20]. Meanwhile, HCV, an RNA virus, has a slightly lower global prevalence, with more than 170 million people with chronic infections [21]. HCV has been shown to be responsible for 27% of the cases of liver cirrhosis and 25% of the HCC cases [20]. The burden of HIV/HCV coinfection has been estimated at 4 to 5 million people worldwide, whereas the prevalence of HIV/HBV coinfection is estimated to be 5% to 7% among HIV-infected individuals (approximately 2 million) [22]. These rates are lower than the rates of 10% to 20% found in highly endemic areas [23-25].
Substance abuse is a significant risk factor for exposure to HIV-1 due to increased high-risk sexual behavior and direct inoculation of the virus into the bloodstream through the sharing of virus-contaminated needles [26]. In addition to enhancing susceptibility to HIV-1 through biological and behavioral means, IVDU that continues once a person is infected has been responsible for more than 36% of AIDS cases; of the 34,233 new AIDS cases in 2009, 6,522 were associated with IVDU [27]. One of the consequences of chronic substance abuse, important in the context of this review, is coinfection with hepatitis. Although opiates are known to modulate the immune system and may augment susceptibility to HIV-1 infection by increasing the number of chemokine receptors present on the cell surface [28] or by increasing viral replication [29], cocaine can facilitate HIV-1 disease progression by impairing macrophage and CD4+ T-cell function and activating HIV-1 gene expression in these cell types as previously reviewed [30], modulating the levels of cytokines and increasing the level of viral replication.
This review examines the effects exerted on the BM by HIV-1 infection and relates these events to the pathogenesis of HIV in the PB and brain. The impact of coinfection with HBV and/or HCV on these interactions and subsequent viral interplay during the course of disease are also reviewed and analyzed. Finally, the impact on HIV-1 infection alone or after coinfection with HCV or HBV within and outside the BM of persistent substance abuse, primarily opiates and cocaine, is discussed.
BM as an Important Reservoir of HIV Infection
HIV-1 likely establishes a number of cellular reservoirs despite the host immune response and therapeutic strategies aimed at decreasing viral load during the primary infection. The BM is one such long-standing reservoir of HIV-1 and has proven to be one of a number of obstacles complicating strategies to eradicate HIV-1 infection. The BM environment consists of HPCs, which are a mixture of fibroblasts, endothelial cells, adipocytes, and myeloid cells [31-33] (Fig 1). HIV-1-infected individuals exhibit many hematologic abnormalities such as anemia, neutropenia, and thrombocytopenia [34, 35] that increase as the disease progresses [36]. These cytopenic conditions may be associated with deficient HPC growth and differentiation [37].
Infection of HPCs [38] and BM stromal cells [39] can lead to altered cytokine profiles and altered differentiation and growth due to the viral proteins gp120, Tat, and Nef [40-42] (Fig. 1B). Many studies have shown that specific lineages of CD34+ HPCs express CD4, CCR5, and/or CXCR4 and that expression of these receptors and coreceptors is required for susceptibility to HIV-1 infection as previously summarized [13]. In an in vitro study, normal human CD34+ HPCs, when incubated with HIV-1 for 24 h, resulted in a noncytopathic infection of the progenitor cells [43]. Similarly, an in vivo study showed that CD34+ HPCs were infected in a subset of seropositive individuals, indicating that these cells may serve as a reservoir within infected individuals [17]. Despite evidence from many studies, the controversy still exists as to whether HPCs can be infected with HIV-1. In this regard, one report has demonstrated that CD34+ HPCs isolated from 10 asymptomatic HIV-1-seropositive individuals were not infected with HIV-1 when tested using conventional and nested polymerase chain reaction techniques [44]. However, it appears that with advanced HIV-1 disease, CD34+ progenitor cells may become more prone to HIV-1 infection. Because this study used asymptomatic patients, the question of susceptibility and permissivity of CD34+ progenitor cells may need to be reevaluated. It has also been reported that the process of reverse transcription is defective in resting cells [45]. Hence it would be difficult to productively infect quiescent progenitor cells [44]. Despite the debate concerning infection of BM progenitor cells, it is believed that even a limited infection of these cells could establish a viral reservoir within this important cellular compartment, particularly over a long period. In this regard, studies have reported the detection of latent HIV-1 genomes present within HPCs isolated from patients who had been treated with highly active antiretroviral therapy (HAART) [46]. These studies have indicated that HPCs are susceptible to HIV-1 infection. Since the infected HPCs have been shown to be long-lived, the virus can be harbored for prolonged periods of time. These studies also demonstrated that HIV-1 gene expression could be activated from latently infected HPCs by treatment with differentiation factors such as phorbol 2-myristate 13-acetate [46] even when patients had been on HAART for more than 6 months prior to isolation of infected HPCs. This observation has suggested that productive viral replication in this cell population could be targeted for elimination by antiretroviral therapy or other therapeutic strategies currently in development. In addition to HPCs, other cell populations found within the stroma of the BM, such as myeloid cells [47] and stromal fibroblasts [39], are also capable of being infected by HIV-1 (Fig. 1B); however, their role in maintaining a latent viral reservoir in the BM is not as well documented. Limited infection of myeloid cells was confirmed by p24 assays as well as by an HIV-1-specific DNA polymerase chain reaction assay. However, no integrated HIV-1 proviral DNA was detected in this cell population, which was devoid of all cellular components found within the BM microenvironment [47]. With respect to HIV infection of BM stromal fibroblasts, the one study that has been performed to date indicated that this subset of cells is also susceptible to infection with HIV-1 and HIV-2 [39]. Consequently, HIV-1 infection of the cells of the BM stroma may facilitate transmission of the virus to HPCs and may also damage the BM microenvironment by dysregulation of the cytokine milieu especially due to an increase in the proinflammatory cytokines within the BM, thereby contributing to abnormal hematopoiesis.
Role of HIV-1-infected BM in HIV-1 CNS Infection and Neurological Dysfunction
HIV-1 infection of the CNS may result in a range of HIV-associated neurocognitive disorders. HIV-1-infected monocytes and lymphocytes can cross the blood-brain barrier (BBB) and transmit virus to target cells within the brain that include perivascular macrophages, microglial cells, and astrocytes [48][49] (Fig. 2). Investigations concerning the etiology of HIV-1-associated dementia (HIVD) have involved sequencing and comparison of HIV-1 gp160 sequences from autopsied bone marrow, lymph node, lung, and four different regions of the brain from a patient suffering from HIVD (44). The comparisons of the sequences demonstrated a closer relationship between viral sequences obtained from the deep white matter of the brain and those derived from the BM than from any other peripheral sources [50]. This observation has suggested that HIV-1-infected cells in the BM (most likely cells within the monocyte-macrophage lineage) migrate out of the BM, enter PB circulation, and traverse the BBB, seeding HIV-1 infection in the brain (Fig. 2). The authors also proposed that trafficking of cells from the infected BM into the brain could accelerate during late stage disease and that this process could probably explain the occurrence of dementia during that time [50]. Changes were also observed in the biological makeup of the BM that may also affect the course of HIV-1 infection in the brain and the process of hematopoiesis and cell departure from the BM [50]. The clival and calvarial regions of the BM (important sites of hematopoiesis), found within the cranium [51], were compared between uninfected and HIV-1-infected individuals. Magnetic resonance imaging analyses were performed to determine if changes within the BM could affect the severity of HIVD. These studies were designed to identify alterations in membranes, membrane permeability, morphological structure, and volume of extracellular spaces within the BM. Variations were found within the clival and calvarial regions that could be a reflection of altered hematopoiesis within the HIV-1-infected BM compartment. These changes correlated with the severity of HIVD observed in the infected individuals [52]. Furthermore, investigations performed in individuals with HIVD have shown that specific subsets of monocytes, the CD14/CD16 subset, were found in increased numbers in AIDS patients [53], and CD69 (monocyte activation marker) was found to be especially increased in individuals with AIDS and dementia [54]. BM-derived macrophages are known to express CD14 [55]; in fact, CD14 and CD16 have been used to identify heterogeneous populations of cells that are trafficking from the BM to various peripheral organs [56, 57]. In an interesting study, HIV-1-infected CD14+CD16+ macrophages were found to accumulate in the CNS of infected individuals with HIV encephalopathy [58], thus strengthening the argument that trafficking of HIV-1-infected cells can enhance the spread of infection. Another recent study identified 5-bromo-2’-deoxyuridine–labeled monocytes originating in the BM that migrated to the CNS in a simian immunodeficiency virus (SIV)-infected CD8+ T-lymphocyte-depleted macaque model. Increased numbers of 5-bromo-2’-deoxyuridine+ monocytes were found in animals that had rapidly progressed to AIDS; this finding was also correlated with soluble CD163, a marker of activated monocytes-macrophages and innate immune activation [59]. This result reinforces the fact that there is increased trafficking of cells from the BM to the brain and that activation of monocytes plays an important role in HIV/SIV neuropathogenesis [15]. It has also been suggested that changes within the cytokine environment of the BM, due to activation of inflammatory responses resulting from HIV-1 infection, can result in an increase of this particular subset of cells and activate the migratory potential of these cells [60]. These alterations could lead to changes in monocyte production and migration from the BM, which can influence the activation state of these cells in circulation. In fact, an increase in the number of activated monocytes has been correlated with HIVD [54].
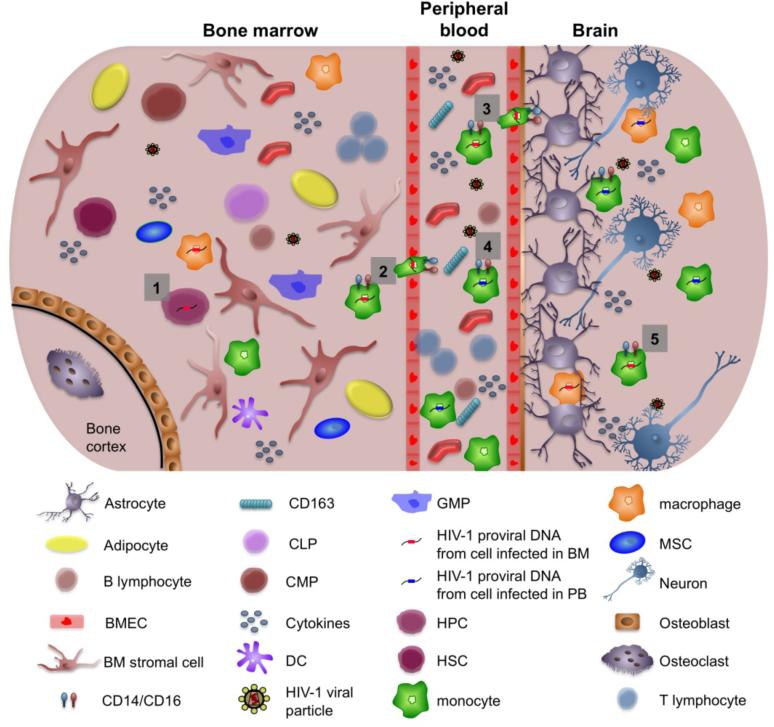
Fig (2).
Migration of human immunodeficiency virus 1 (HIV-1)-infected cells from the bone marrow (BM) to the central nervous system (CNS). (1) Hematopoietic progenitor cells (HPCs) expressing CD4 and CXCR4/CCR5 can be infected in the BM of HIV-1-infected patients. (2) HIV-1-infected monocytes and macrophages from the bone marrow reseed the peripheral blood (PB). (3) HIV-1-infected cells from the BM that have reseeded the peripheral blood traverse the blood-brain barrier as well as (4) monocytic cells infected within the peripheral blood compartment. These cells have been shown to be more CD14+/16+ and associated with increased neurological disease. (5) Both monocytic cells that were infected in the BM and cells infected in the peripheral blood contribute to carrying HIV into the CNS, thereby spreading infection to cells found within the CNS such as perivascular macrophages, microglial cells, and astrocytes. Changes in cytokine profiles brought about by HIV-1 infection can also affect the migration of HIV-1-infected cells, thus helping to enhance the progression of HIV-1 infection. In addition, soluble CD163 found within the peripheral blood has been associated with HIV-1-associated neurological disease. BMEC, bone marrow microvascular endothelial cell; CLP, committed lymphoid progenitors; CMP, committed myeloid progenitors; DC, dendritic cells; GMP, granuloctye-macrophage progenitors; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells.
Impact of HBV and/or HCV on BM
The primary site of the pathogenic impact of HBV occurs in the liver with secondary effects observed in other organs such as pancreas, kidney, skin, spleen, and cells of the BM [61-68]. HBV has also been detected in peripheral blood mononuclear cells (PBMCs) and polymorphonuclear leukocytes [69, 70]. With regard to the BM, acute HBV infection can lead to mild depression in a number of physiological properties, such as hematopoiesis [71]. One such study demonstrated that serum containing HBV DNA inhibited the differentiation process and proliferation of BM progenitor cells such as colony forming unit-granulocytes, erythrocytes, monocytes, and megakaryocytes, colony-forming unit-granulocytes/macrophages, burst forming unit-erythroids, and colony-forming unit-erythroids [72] (Fig. 1C). The inhibition of colony formation was reversed under conditions in which the serum was devoid of viral particles. HBV-induced inhibition of colony formation was also neutralized by anti-HBV murine monoclonal antibodies, indicating that the presence of HBV virions was responsible for the suppression of the differentiation process. An additional study confirmed that the degree to which differentiation and proliferation were inhibited was proportional to the multiplicity of infection of HBV used in the study [73].
HCV is considered to be an important factor to consider for the development of hematologic abnormalities, which can range from immune thrombocytopenia to lymphoma [74]. In fact, thrombocytopenia has often been associated with chronic liver disease and has also been identified in patients infected with HCV [75], possibly involving BM suppression with decreased platelet production and diminished levels of the hematopoietic growth factor thrombopoietin (Fig. 1D). Thrombopoietin is produced in the liver, BM, and kidney and has been shown to be responsible for regulating megakaryocyte development and maturation, along with platelet production and release [76, 77], thereby playing an important role in the process of hematopoiesis.
Impact of HBV and/or HCV on Peripheral Immune Function
Although HBV and HCV can affect BM development, they demonstrate a direct effect on peripheral immune cells, immune activation, and immunoregulatory pathways, suggesting a comprehensive impact on the immune system. In an interesting study, DNA microarray analysis was performed on PBMCs in groups of individuals who were infected either with HIV or HCV or coinfected to delineate the differential gene expression patterns in PBMCs [78]. HCV exhibited a distinct immunologic profile with an increase in the proinflammatory immune response in non-T cells, which was in contrast to HIV, which induced an immune profile with activation of CD4+ and CD8+ T cells. Expression levels of genes associated with various receptors on natural killer cells, B cells, and plasmacytoid dendritic cells were increased in HCV-infected individuals when compared to levels in HIV-1-infected individuals. Expression of the proinflammatory cytokine, fractalkine, was also elevated in the PB in the HCV-infected group, suggestive of liver fibrosis or injury in contrast to levels found in the HIV-1-infected group. This study presented an in-depth comparison of the different ways host immune responses resulting from chronic HCV and HIV infections [78] are regulated. Similarly, efforts are under way to determine the effects of chronic HBV infection on the function of the peripheral immune system. A study involving 206 individuals with chronic HBV infection demonstrated that T-cell dysfunction in these individuals was extensive and persisted with a significant decrease in CD3+ T cells, which translated into a loss of competent cells that were immunologically capable of challenging the infection [79]. In fact, there have been reports that antiviral treatment can increase CD4+ [80] and CD8+ [81, 82] T-cell responsiveness to chronic HBV infection, suggesting that these cell populations were present but suppressed by HBV infection. Thus, HBV-induced T-cell loss will facilitate viral persistence and continued chronic viral infection.
Impact of HIV-1 Infection on Hepatitis Virus Pathogenesis
The natural history of infections is often altered in people coinfected with HIV and HBV and/or HCV. Indeed, in individuals undergoing long-term effective management of HIV disease, it is often the viral-induced hepatitis in a coinfected individual that becomes the dominant disease [83]. In fact, similar studies for HIV and HBV/HCV coinfections have shown the risk of liver-related deaths to be 2 to 3 times higher in individuals coinfected with HIV-HBV/HCV compared with those monoinfected with HBV/HCV [84]. Taken together, however, it appears that HIV has more of an effect on HBV disease than HBV does in modifying HIV pathogenesis, although some studies report an increase in the progression of HIV to AIDS in people with serological markers of HBV [85].
Although icteric disease may follow a more moderate course, individuals coinfected with HIV and HBV or HCV often experience a more aggressive chronic liver disease, with accelerated development of fibrosis and cirrhosis [86] (Fig. 3). HIV-1 can impact the natural course of HBV infection and leave the HIV-1-infected population vulnerable to development of chronic hepatitis B compared to those individuals who have not been infected with HIV-1 [87, 88]. Hadler et al. observed a threefold increase in the risk of developing chronic HBV infection in unvaccinated individuals who were infected with HIV-1 before being infected with HBV; they also did not observe any subsequent effect on liver enzymes or even on the severity of clinical illness [88]. The increase in HBV carriage could have been due to the fact that clearance of HBV infection was dependent on normal functioning of the T helper cells, which was affected during HIV-1 infection [88]. Thus, the detrimental impact of HIV-1 on the immune system could lead to a defect in the resolution of acute HBV infection, enabling chronic HBV infection to develop more readily.
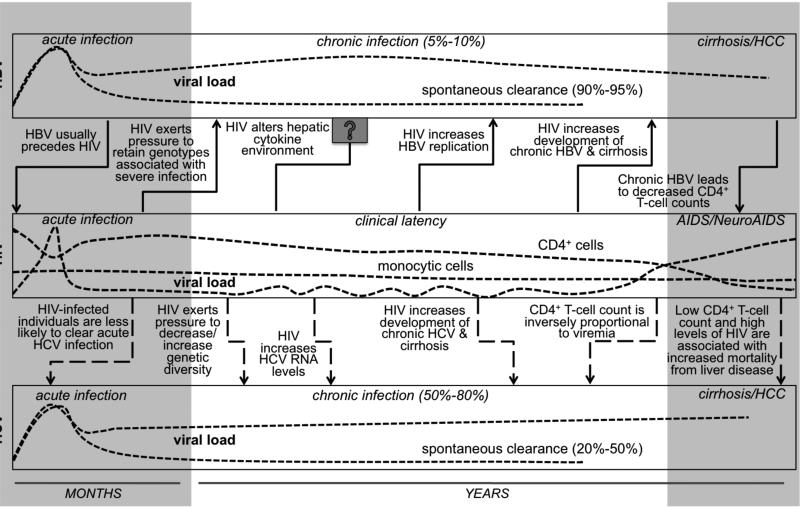
Fig. (3). Effect of human immunodeficiency virus (HIV) on hepatitis pathogenesis.
HIV can affect the natural history of hepatitis B virus (HBV) and hepatitis C virus (HCV) pathogenesis. HIV has been identified as a risk factor for increased probability of developing hepatocellular carcinoma (HCC). It leads to accelerated fibrosis and cirrhosis and also to increasing viral load (HBV) and RNA levels (HCV). It is still unclear whether HBV and HCV exert effects on HIV-1 pathogenesis. AIDS, acquired immunodeficiency syndrome.
HIV-1 accelerates the progression of HBV-associated liver disease toward cirrhosis and is usually associated with a decrease in the size and quality of the CD4+ T-cell compartment [89]. Lower CD4+ T-cell counts are often associated with increased risk for HCC in HIV-HBV coinfected individuals (Fig. 3). However, it remains unclear as to whether HIV-1 mono-infection can increase the risk of HCC [90]. Also, HBV-induced liver disease, which is an immune-mediated process, is accelerated by HIV-1. This event may be due to the cytopathic effects induced by the HIV-1-encoded proteins rather than by the immune system itself. One such form of liver disease is known as fibrosing cholestatic hepatitis [91]. HBV has been shown to exist in at least eight different genotypes (A – H), and many studies have shown that specific HBV genotypes correlate with disease severity, development of acute versus chronic infection, and progression to HCC as previously reviewed [92]. A recent study has shown the prevalence of dual infection of HBV genotypes A+G in 17.6% of HIV-1/HBV coinfected individuals [93]. In this study, A+G dual genotype infections correlated with increased HIV viral load [93]. The effect of A+G dual genotype infections within the context of HIV-1 infection has not yet been determined, but genotype D has been shown to be involved in liver fibrosis especially in immunocompromised individuals [94].
Liver disease in chronic HBV patients has not only been associated with the presence of specific genotypes, as indicated above, but also with mutations within the precore and core regions of the HBV genome [95, 96] that appear to arise during the course of chronic infection. These mutations have been found in HBV monoinfected patients who had progressive liver disease, who were on immunosuppressive drug therapy [96], and who were associated with enhanced virulence and, in some cases, enhanced replication of the virus [97]. It is possible that certain precore/core region HBV mutants are more prevalent in individuals coinfected with HIV-1/HBV than in monoinfected individuals. In this regard, HBV core deletion mutants are known to be associated with aggressive liver disease, so the presence of this core region mutant could be responsible, in part, for accelerated liver disease in individuals coinfected with HIV-1/HBV [98]. Thus, HIV could be responsible for placing selective genetic pressure on HBV that results in the enrichment of specific HBV mutants that may be involved in causing more severe HBV infection (Fig. 3). The changes in cytokine profiles during the course of coinfection may also be involved in the etiology of more severe liver disease.
HIV-1 infection also affects the HCV life cycle, resulting in a more progressive form of liver disease [19]. This situation is likely attributable to the increased HCV viral load found in the population coinfected with HIV-1/HCV compared to the population monoinfected with either HCV or HIV-1 [99-101]. During acute HCV infection, it is imperative that T cells clear the infection to prevent the development of a chronic state of HCV infection [102]. As previously demonstrated with HBV-infected individuals, immune suppression in HIV-1-infected individuals allows acute HCV infection to develop into a chronic state of infection (Fig. 3) because CD4+ and CD8+ T-cell responses are significantly reduced in these individuals [103]. HCV has six genotypes and multiple subtypes, each associated with specific clinical implications, as reviewed previously [103]. A small study analyzed the HCV genetic diversity using multiple HCV clones at two different time points from individuals coinfected with HIV-1/HCV using only HCV genotype 1a-positive patients. The clones examined represented two different regions of the HCV genome, the hypervariable region 1 (HVR-1) and the nonstructural protein 3, which have been shown to encode for epitopes that are targets for neutralizing antibodies and cellular immune responses, respectively. Decreased HCV genetic diversity was observed in the clones obtained from the coinfected patients compared to the clones from HCV monoinfected patients. The authors suggested that the decline in genetic diversity could be attributable to the changes found in the immune responses, particularly the low CD4 counts brought about by infection with HIV-1 [104]. Although there have been conflicting studies in the past that have shown increased genetic diversity in the HVR-1 region of HCV in clones obtained from patients coinfected with HIV-1/HCV, not all of the patients were infected with the same genotype of HCV, which may also account, at least in part, for the results obtained in these studies [105].
HBV/HCV coinfection with HIV-1 has also been associated with increased morbidity and mortality compared to monoinfection with either HBV or HCV [19, 84]. In addition to immune suppression, HIV can also alter HBV or HCV pathogenesis as a result of an intrahepatic interaction between the viruses. Viral proteins such as gp120 can interact with hepatitis gene products and can affect hepatocytes via interactions with the chemokine receptors CXCR4 and CCR5, leading to an increase in viral replication. Some pathogenic mechanisms have been identified that result from the interaction of HIV-1 with HCV. It is possible that HIV-1 can infect hepatic stellate cells, activating them and leading to increased fibrosis [19]. HIV-1 affects the natural course of hepatitis infection and of treatment regimens for chronic hepatitis infection. However, the exact molecular mechanisms by which it does so remain unclear, warranting additional studies in this area.
The above-mentioned studies provide ample evidence that HIV-1 affects and accelerates hepatitis disease progression with increased viral loads and establishment of liver diseases such as fibrosis and cirrhosis. Both of these conditions are important pathological intermediates leading to the development of HCC. Although HIV-1 is not routinely considered to be an oncogenic virus, its detrimental effect on the immune system has led to increased risk of infection with pathogens that do cause cancer, for example, cervical cancer due to human papilloma virus infection, non-Hodgkins lymphoma due to Epstein-Barr virus, and Kaposi's sarcoma due to human herpes virus type 8 [106]. Similarly, HIV-1 does not allow for clearance of acute hepatitis infection, causing an increased frequency of chronic hepatitis infections. The acute hepatitis infection will ultimately lead to cirrhosis, which will increase the probability of developing HCC. Thus, there is an indirect, but definite, correlation between coinfection with HIV-1/HBV or HCV and increased risk for HCC. A number of cohort studies investigated the incidence rates of HCC in individuals coinfected with HIV-1/hepatitis. One such report was a retrospective cohort study of 16,439 HIV-1-infected US veterans, of which 4,761 were coinfected with HIV-1 and HCV. When they divided their cohort into pre-HAART versus those who received HAART, they found that HCV coinfection with HIV-1 increased the risk of cirrhosis 10-fold in the pre-HAART era whereas in the HAART era it increased the risk approximately 20-fold; the risk for HCC increased 5-fold [107]. Another retrospective study of 14,018 HIV-1-infected US veterans demonstrated that these individuals had a higher prevalence of HCC compared to HIV-1-negative individuals. The increased prevalence of HCC could be associated with HCV coinfection and/or alcohol abuse [108]. A smaller study involving 2,383 individuals infected with HIV-1 also identified a higher incidence rate of HCC compared to that of the general population. Of six cases of HCC, four were in individuals coinfected with HCV and two were in individuals with a history of alcohol abuse [109]. With HAART treatment, there is an improvement in the survival of coinfected individuals, and the increased life expectancy can lead to an increase in the emergence of cases of HCC and end-stage liver disease due to coinfection with HCV and HIV-1. Other chronic conditions that develop as comorbidities in HIV-1-infected patients such as type II diabetes mellitus, metabolic syndrome, and obesity, are also emerging as HCC risk factors in the general population [110-112]. With HAART therapy extending the lifespan of HIV-1-infected individuals, these conditions are expected to become much more prevalent, and there remains the potential that viral hepatitis may exacerbate this effect. Other studies have also reported a trend toward increased numbers of HCC cases over time [113]. In these studies, this effect was adjusted for age suggesting that the overall increase in the incidence over time was even greater, with the reported risk in older adults aged 50 and greater 10 times higher than those between 30-39 years of age. An increase in the number of HCC cases in the future, within the context of the aging HIV-infected population, has the potential to greatly increase the total number of HCC cases reported overall [113]. This again demonstrates another indirect effect of HAART treatment on the development of HCC, however in the context of increased lifespan. Although these observations suggests interplay between HIV-1 and HCV, it is necessary also to determine the effect of HAART treatment in the coinfected patients. HAART, which is known to be hepatotoxic [114], could increase liver injury and this toxicity could potentially contribute to HCC, as documented in a study in which HIV-HCV coinfected individuals on HAART were frequently diagnosed as having coexistent cholestatic and cytolytic hepatotoxicity [114]. An interesting question that remains unanswered is the mechanism via which HAART affects the liver of an HIV/HCV coinfected individual. To address this question, a cohort of 39 HIV/HCV coinfected individuals were examined for their immune status and its association with severity of liver disease [115]. Patients with low or undetectable viral load and a CD4+ cell count of >200 cells were more at risk for cirrhosis as compared to the individuals with lower CD4+ cell counts or higher viral load [115]. An explanation for this phenomenon could be attributed to HAART facilitating the restoration of CD4+ T cell counts by rescuing viral load and restoring immune system responses, which could adversely impact the severity of HCV disease [115]. While HAART is being regarded as one of the leading causes of liver-related mortality in HIV-infected individuals [116], it obviously needs to be utilized for keeping HIV-1 infection under control. Given this, several studies have begun to try and understand whether the potential hepatotoxic effects of HAART can be minimized in HIV-1 and HBV/HCV coinfected individuals. These studies have shown in coinfected individuals that treatment of viral hepatitis can be targeted with hepatitis-specific antivirals, leading to a significant reduction or regression [117, 118] in liver fibrosis progression, while suppressing HBV and HCV (reviewed in [119]). Antiretroviral drugs such as nevirapine, that are now being included in the HAART regimen, show a favorable liver safety bioprofile [120]. These drugs provide HIV control, increased CD4+ cell count, which can lead to a positive benefit to the liver of a HIV/HCV coinfected individual [121]. Several retrospective studies of HIV/HCV coinfected individuals, have examined liver biopsies and have found that slower progression of liver fibrosis is associated with the use of HAART, suppression of HIV and higher CD4+ cell counts [120,122][123]. The current consensus is that initiation of HAART including the antiretrovirals with a favorable biosafety profile, at an early stage is favorable as it may preserve the immune system, while working at controlling HIV infection and thus, protecting the health of the liver [121, 124].
Impact of Substance Abuse on HIV Disease within the BM
A large population of HIV-1-infected individuals is known to abuse a variety of drugs, with almost one-third of the individuals with AIDS frequently abusing opioids [125]. Considerable evidence exists that opioids modulate the function of the immune system [126, 127]. In addition to being expressed within the CNS, opioid receptors are also expressed by primary human CD34+ HPCs. Specifically, within CD34+ cells, the μ-opioid receptor (MOR-1) was identified on the immature CD38dim (low to negative expression of CD38) and CD38bright (high expression of CD38) cell subpopulations, whereas the fully differentiated blood cells lacked MOR-1. This observation suggests that MOR-1 plays a role in the differentiation process of early CD34+ stem and progenitor cells [128]. In addition, the expression of MOR-1 has been demonstrated on PB and cord blood CD34+ cells with increased levels of the receptor detected on immature cord blood CD34+ cells. Activation of MOR-1 by the endogenous opioid enkephalin or the exogenous opioid morphine (Fig. 4) induces the MAPK pathway, which has been shown to induce processes associated with cellular proliferation and differentiation [129]. Morphine has been shown to result in a significant decrease in the number and proportion of CD4+/CD8+ double-positive cells and in an increase in the populations of CD4+/CD8-, CD4-/CD8+, and CD4-/CD8- double-negative cells leading to morphine-induced thymic hypoplasia in mice [130]. In vitro chronic morphine exposure has also been implicated in CD4+ T-cell Th2 differentiation [131, 132]. This finding was confirmed in vivo when mice were implanted with morphine pellets, exposing them to morphine for 72 h, resulting in a cytokine profile that was consistent with CD4+ T-cell Th2 differentiation [132]. Thus, MOR-1 agonists, both endogenous (enkephalins) and exogenous (morphine), may be imperative factors to consider for hematopoietic stem cell differentiation and proliferation (Fig. 4). Cocaine is another powerful addictive CNS stimulant, abused by a significant number of HIV-1-infected individuals, and is responsible for eliciting various alterations in the functions of multiple organs [133]. No studies have reported any effect of cocaine on HIV disease within the BM or during BM-mediated erythropoiesis [134]. However, a large number of studies have shown the significance of cocaine in modulating the functions of the immune system [135, 136]. Such studies have demonstrated in an HIV-1-infected humanized PB leukocyte (huPBL)-severe combined immunodeficient (SCID) mouse model, wherein systemic cocaine administration led to accelerated HIV-1 infection of huPBLs, a decrease in CD4+ T cells [137] and a dramatic rise in viral load that eventually led to an accelerated form of HIV disease [138].
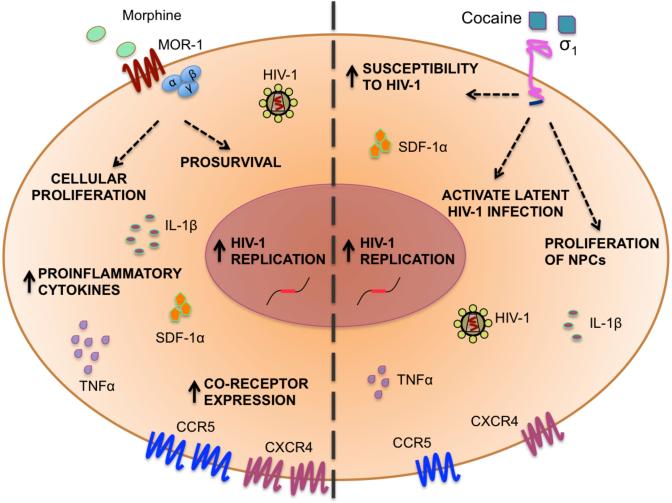
Fig. (4). Impact of opioids and cocaine on human immunodeficiency virus (HIV) disease.
Opioids such as morphine, an agonist for the μ-opioid receptor (MOR-1), have a variety of effects on HIV to exacerbate disease progression including increased replication of HIV-1 and altered cytokine profiles, which can prove to be detrimental to the central nervous system. Morphine can upregulate coreceptor expression levels, have a prosurvival effect on cells, and can act as an inducer of cell proliferation. Cocaine, a sigma1 receptor agonist, can increase susceptibility of peripheral blood leukocytes to HIV-1, decrease proliferation of neural progenitor cells (NPCs), activate latent HIV-1 infection, and increase cytokine profiles. IL, interleukin; SDF, stromal cell-derived factor; TNF, tumor necrosis factor.
Effects of Substance Abuse on HIV-1 Pathogenesis and Hepatitis Coinfection
Substances of abuse add another complex dimension to the already complicated effects associated with HIV-1 and hepatitis coinfection. Morphine, the most active metabolite of heroin, has been shown to induce many biological effects including cell survival or proliferation (Fig. 4). However, the exact molecular mechanisms involved in these processes have yet to be established [139]. Evidence from many studies has suggested that opioids play an important role in HIV-1 pathogenesis. Opioids interact with the cell surface receptor MOR-1 (Fig. 4), and this interaction can stimulate cells. After the interaction of the drug and receptor, MOR-1 can then trigger a signaling cascade leading to an alteration in viral gene expression, thus affecting HIV-1 infection and replication [140]. Morphine-treated SIV-infected lymphocytes have been known to survive for longer periods of time, indicating that opioids exert a protective effect on cells from apoptosis induced by SIV, thus allowing continued viral proliferation [141, 142]. Studies have shown that PBMCs treated with morphine exhibited an increase in the level of HIV-1 replication [143] (Fig. 4). Morphine was also responsible for amplifying HIV-1 gene expression in chronically HIV-1-infected promonocytes cocultured with lipopolysaccharide-stimulated human brain cells [144]. In addition to altering the immune system, opioids can also affect the CNS directly, causing neurological dysfunction. HIV-1 Tat and morphine act cooperatively, leading to activation of microglial cells, which, when activated, can secrete proinflammatory cytokines that are detrimental to neurons and cause the upregulation of the chemokine receptor CCR5, thus exacerbating HIV-1 pathogenesis [145] (Fig. 4). Infection of rhesus macaques with the HIV/SIV chimeric strains SHIVKU-1B, SHIV89.6P, and SHIV17E-Fr resulted in the establishment of a higher viral set point and increased viral replication in the CNS when the monkeys were exposed to chronic doses of morphine [146]. However, there is a paucity of information concerning the effects of opioids on HBV/HCV infection within the BM and on the process of hematopoiesis. This gap in knowledge needs to be addressed.
Cocaine can modulate the immune system by depressing it [147], which can result in progression to AIDS; it can also increase the risk for developing secondary opportunistic infections [137]. The sigma1 receptor is responsible for mediating many of the acute and chronic effects of cocaine abuse [148]. Previous studies using the SCID mouse model have shown the effects of cocaine on HIV-1 replication. The SCID mice were implanted with huPBLs, infected with HIV-1, and given cocaine. The huPBLs from cocaine-treated animals were more susceptible to HIV-1 infection than were animals exposed to cocaine (Fig. 4). Exposure to cocaine was associated with an increase in viral load and a lower CD4:CD8 ratio. This in vivo study also confirmed the negative relationship between cocaine use and HIV-1 pathogenesis and disease progression [137]. Perivascular macrophages and microglial cells are major target cell populations in the brain for HIV-1 infection [60]; however, the virus does not infect neurons, though indirectly the virus and/or viral proteins (gp120, Tat, and Vpr) may lead to neuronal loss and HIVD [149-152]. Not only does cocaine impact the immune system, but chronic cocaine exposure can also lead to a decrease in the proliferation of neural progenitor cells, thus targeting the CNS through yet another pathological pathway as previously reviewed [153] (Fig. 4). Therefore, HIV-1 and cocaine can both have a detrimental effect on the CNS. Cocaine can also have an important effect on latent HIV-1 infection in that it can activate viral replication. Buch et al. have shown increased HIV-1 replication in cocaine-treated human monocyte-derived macrophages. Cocaine treatment could also activate latent infection within a promonocytic cell line (Fig. 4), suggesting that HIV-1 patients abusing cocaine could have an increased severity of HIV-1 infection and accelerated progression toward HIVD [30].
Cohort studies are complex due to the various biological interactions that occur within humans while having to control for variables such as cohort demographics, adherence to HAART, attrition etc., and hence, a multi-pronged approach along with in vitro studies and in vivo mouse model studies are warranted to understand the effects of specific drugs of abuse on HIV-1 pathogenesis. However, a number of cohort studies have clearly demonstrated an association of cocaine abuse and acceleration of HIV disease [154-156]. A 30-month longitudinal study of 222 HIV-1-seropositive active substance-abusers, showed a significant elevation in the viral loads of individuals who were crack-cocaine users independent of HAART [154]. A lower proportion of the crack-cocaine users showed a higher but controlled viral load but these individuals were also on HAART, thus, implicating the lack of adherence to therapy as a risk factor for HIV disease progression, among substance-abusers [154]. Additionally, it was also demonstrated that the active drug-using cohort had a significant association with accelerated decline in CD4+ cell counts, thus accounting for accelerated disease progression [154]. Another study, which was a six-center national cohort of 1686 HIV-1-infected women who were non-drug users and 483 crack-cocaine-abusing HIV-1-infected women, examined patterns of crack-cocaine use and its association with several parameters of HIV disease, including CD4+ T lymphocytes and viral RNA. This prospective study of crack-cocaine users showed greater CD4+ cell loss and higher viral RNA levels in the using population [155]. While, both of these studies compared the effect of cocaine on HIV-1-infected individuals, another study comparing 80 HIV-1 seropositive and 42 seronegative crack-cocaine smoking African-American women, demonstrated that CD4+ cell counts were lower for a given viral load as cocaine-doses increased, and it was concluded that this was due to the effects of cocaine on viral load and CD4+ cells [156].
A number of studies and reviews have reported and discussed the prevalence of HIV-1 and hepatitis coinfection in a substance-abusing population [157-160]; however, few of these studies considered the molecular effects of substance abuse on the two infections [161, 162]. A recent study evaluated liver fibrosis among 497 patients, including HCV-monoinfected and HIV/HCV-coinfected substance abusers for whom opiates and cocaine were the dominant drugs of choice. Advanced liver fibrosis was three- to five-fold more prevalent in coinfected patients than in patients with HCV monoinfection [161]. Although the investigators did not comment on the molecular mechanisms driving advanced liver fibrosis, it is possible that HIV and substance abuse are partially involved in the etiology of the advanced stage of fibrosis. Another study explored the possible effects of IVDU on immune responses in HCV-monoinfected and HIV/HCV-coinfected individuals who were segregated into substance abusing and nonusing groups. The group with coinfection and concurrent substance abuse had significantly higher interferon-γ and interleukin-10 HCV-specific responses than the coinfected nonuser group [162]. The same investigators had previously shown that higher interferon-γ responses in coinfected individuals correlated with lower liver inflammation and fibrosis scores [163], thus contradicting results from other studies. With respect to HIV/HBV coinfection however, there is a general lack of investigations that examine the impact of substance abuse on the immune responses in HIV/HBV coinfection, further suggesting that detailed, systematic research is needed to rigorously define the impact of substance abuse on HIV-1 and HBV/HCV mono- and coinfection. Despite the lack of knowledge concerning the molecular mechanisms that drive these results, it is clear that substance abuse emerges as a key player in modifying immune responses during the course of viral disease.
DISCUSSION
The BM plays a vital role in the pathogenesis of HIV-1 because it serves as one of the reservoirs for latent HIV-1 infection, which when activated can spread from the BM to distant sites including the brain and other end organs. HIV-1 in turn adversely affects the process of hematopoiesis and the cytokine environment within the BM. Infection of the cells of the BM can further affect and seed infection to other end organs, but its effects are particularly well-defined in the CNS. Various studies have shown the similarities between viral sequences found within the BM and the CNS, providing evidence for the migration of HIV-1-infected BM cells across the BBB, thereby spreading infection into the brain. HIV-1 is not the only virus to impact the BM environment; coinfection with members of the hepatitis virus family is also known to have a depressive effect on the differentiation and proliferation of cells within the BM compartment. Although there is ample information concerning the detrimental effects of HBV and HCV on BM function, there is a dearth of knowledge relative to the effects of other hepatitis viruses such as hepatitis A virus. The effects of hepatitis are not localized only to the BM but also extend to the PB compartment and the peripheral immune system, significantly impacting PBMCs and the T-cell populations.
Coinfections with HIV and hepatitis have harmful effects on the body. Interestingly, HIV-1 seems to have a considerable impact on the pathogenesis of hepatitis but hepatitis does not seem to have a significant impact on HIV-1 pathogenesis. Coinfected individuals usually develop a more hostile and accelerated form of liver disease, such as fibrosis and cirrhosis, eventually leading to the development of HCC. It is still debated whether HIV-1 monoinfection can be considered a risk factor for development of HCC. However, there are enough studies to prove that it definitely remains a strong risk factor when present during coinfection with hepatitis.
Although HIV-1 alters cell function in a number of cellular and tissue compartments and may alter the natural history of a number of coinfecting pathogens, including members of the hepatitis virus family, factors such as substance abuse and coinfections may alter the course of HIV-1 pathogenesis and disease. Studies concerning chronic opioid exposure and cocaine abuse during the course of HIV-1 infection have shown higher viral loads, increased viral replication, and alterations of cytokine levels within the infected cell populations. However, little is known about the effect of substance abuse on the biological makeup of BM or on HIV-1 infection within the BM. Studies targeting this gap in knowledge will help define the mechanistic basis for chronic substance abuse-mediated alterations in HIV-1-infected cell physiology and trafficking of cells from the BM compartment into the peripheral circulation. Results from these investigations will be important for understanding the impact that substance abuse has on HIV-1 pathogenesis and may facilitate the design of more effective therapeutic strategies for HIV/AIDS.
ACKNOWLEDGEMENTS
Drs. Michael Nonnemacher, Vanessa Pirrone, and Brian Wigdahl are supported in part by funds from the Public Health Service, National Institutes of Health through grants from the National Institute of Neurological Disorders and Stroke, NS32092 and NS46263 (Dr. Brian Wigdahl, Principal Investigator), and the National Institute of Drug Abuse, DA19807 (Dr. Brian Wigdahl, Principal Investigator). Dr. Michael Nonnemacher is also supported by research developmental funding provided by the Department of Microbiology and Immunology and the Institute for Molecular Medicine and Infectious Disease, Drexel University College of Medicine. Dr. Anand Mehta is supported by grants R01 CA120206 and R01 CA136607 from the National Cancer Institute (NCI), the Hepatitis B Foundation, and an appropriation from The Commonwealth of Pennsylvania. Dr. Timothy Block is supported by grants R01 CA136607 and R01 CA120206 from NCI.
LIST OF ABBREVIATIONS
- AIDS
acquired immune deficiency syndrome
- BBB
blood-brain barrier
- BM
bone marrow
- CNS
central nervous system
- HAART
highly active antiretroviral therapy
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV-1
human immunodeficiency virus type 1
- HIVD
HIV-1-associated dementia
- HPCs
hematopoietic progenitor cells
- huPBL
humanized PB leukocyte
- IVDU
intravenous drug use
- MOR-1
μ-opioid receptor
- PB
peripheral blood
- PBMCs
peripheral blood mononuclear cells
- SCID
severe combined immunodeficient
- SIV
simian immunodeficiency virus
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest to report.
References
- 1.2010 Report on the global AIDS epidemic. UNAIDS/WHO; 2010. [Google Scholar]
- 2.Bartlett JaFA. The Guide to Living with HIV Infection. Sixth ed A Johns Hopkins Press Health Book; [Google Scholar]
- 3.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998 Apr 17;280(5362):427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 5.Fierer DS, Klotman ME. Kidney and central nervous system as reservoirs of HIV infection. Curr Opin HIV AIDS. 2006 Mar;1(2):115–20. doi: 10.1097/01.COH.0000209581.88166.89. [DOI] [PubMed] [Google Scholar]
- 6.Gunthard HF, Havlir DV, Fiscus S, Zhang ZQ, Eron J, Mellors J, et al. Residual human immunodeficiency virus (HIV) Type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J Infect Dis. 2001 May 1;183(9):1318–27. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- 7.McElrath MJ, Pruett JE, Cohn ZA. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci U S A. 1989 Jan;86(2):675–9. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilareski EM, Shah S, Nonnemacher MR, Wigdahl B. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009;6:118. doi: 10.1186/1742-4690-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997 May 8;387(6629):183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 10.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–93. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 11.Siliciano RF. What do we need to do to cure HIV infection. Top HIV Med. 2010 Aug-Sep;18(3):104–8. [PubMed] [Google Scholar]
- 12.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011 Sep 19; doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 13.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008 Sep;6(5):388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003 Nov;74(5):650–6. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 15.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010 Apr;6(4):e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr Opin HIV AIDS. 2011 Jan;6(1):43–8. doi: 10.1097/COH.0b013e32834086b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley SK, Kessler SW, Justement JS, Schnittman SM, Greenhouse JJ, Brown CC, et al. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992 Jul 15;149(2):689–97. [PubMed] [Google Scholar]
- 18.McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antivir Ther. 2007;12(Suppl 3):H3–13. [PubMed] [Google Scholar]
- 19.Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol. 2009 Aug;83(15):7366–74. doi: 10.1128/JVI.00191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006 Oct;45(4):529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007 May 7;13(17):2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl):S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Ko NY, Lee NY, Chang CM, Ko WC. Seroprevalence of viral hepatitis and sexually transmitted disease among adults with recently diagnosed HIV infection in Southern Taiwan, 2000-2005: upsurge in hepatitis C virus infections among injection drug users. J Formos Med Assoc. 2008 May;107(5):404–11. doi: 10.1016/S0929-6646(08)60106-0. [DOI] [PubMed] [Google Scholar]
- 24.Nyirenda M, Beadsworth MB, Stephany P, Hart CA, Hart IJ, Munthali C, et al. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. J Infect. 2008 Jul;57(1):72–7. doi: 10.1016/j.jinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Diop-Ndiaye H, Toure-Kane C, Etard JF, Lo G, Diaw P, Ngom-Gueye NF, et al. Hepatitis B, C seroprevalence and delta viruses in HIV-1 Senegalese patients at HAART initiation (retrospective study). J Med Virol. 2008 Aug;80(8):1332–6. doi: 10.1002/jmv.21236. [DOI] [PubMed] [Google Scholar]
- 26.KJWR Do drugs of abuse impact on HIV disease? J Neuroimmunol. 2004;147:6–8. doi: 10.1016/j.jneuroim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Drug-Associated HIV Transmission Continues in the United States. Centers for Disease Control and Prevention; 2002. [Google Scholar]
- 28.Anthony ICAJ, Stephens B, Bell JE. The effets of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- 29.DRV D. Opiates as potential cofactors in progression of HIV-1 ifections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007 Dec;13(6):483–95. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 31.K. D. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–37. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- 32.Allen TD, Dexter TM. The essential cells of the hemopoietic microenvironment. Exp Hematol. 1984 Aug;12(7):517–21. [PubMed] [Google Scholar]
- 33.Liesveld JL, Abboud CN, Duerst RE, Ryan DH, Brennan JK, Lichtman MA. Characterization of human marrow stromal cells: role in progenitor cell binding and granulopoiesis. Blood. 1989 May 15;73(7):1794–800. [PubMed] [Google Scholar]
- 34.Aboulafia DM, Mitsuyasu RT. Hematologic abnormalities in AIDS. Hematol Oncol Clin North Am. 1991 Apr;5(2):195–214. [PubMed] [Google Scholar]
- 35.Davis BR, Zauli G. Effect of human immunodeficiency virus infection on haematopoiesis. Baillieres Clin Haematol. 1995 Mar;8(1):113–30. doi: 10.1016/s0950-3536(05)80234-3. [DOI] [PubMed] [Google Scholar]
- 36.Scadden DT, ZLaGJ Pathophysiology and management of HIV-associated hematologic disorders. Blood. 1989;74:1455. [PubMed] [Google Scholar]
- 37.Re MC, FG, Zauli G, La Placa M. Human immunodeficiency virus type 1 (HIV-1) and human progenitor cells. Arch Virol. 1994;137(1-2):1–23. doi: 10.1007/BF01311169. [DOI] [PubMed] [Google Scholar]
- 38.Leiderman IZ, GM, Adelsberg BR, Siegal FP. A glycoprotein inhibitor of in vitro granulopoiesis associated with AIDS. Blood. 1987;70(5):1267–72. [PubMed] [Google Scholar]
- 39.Scadden DT, Zeira M, Woon A, Wang Z, Schieve L, Ikeuchi K, et al. Human immunodeficiency virus infection of human bone marrow stromal fibroblasts. Blood. 1990 Jul 15;76(2):317–22. [PubMed] [Google Scholar]
- 40.Sugiura K, ON, Pahwa R, Kalyanaraman VS, Pahwa S. Effect of human immunodeficiency virus-1 envelope glycoprotein on in vitro hematpoiesis of umbilical cord blood. Blood. 1992;80(6):1463–9. [PubMed] [Google Scholar]
- 41.Zauli G, RM, Visani G, Furlini G, La Placa M. Inhibitory effect of HIV-1 envelope glycoproteins gp120 and gp160 on the in vitro growth of enriched (CD34+) hematopoietic progenitor cells. Arch Virol. 1992;122(3-4):271–80. doi: 10.1007/BF01317189. [DOI] [PubMed] [Google Scholar]
- 42.Calenda V, GP, Delamarter JF, Chermann JC. Involvement of HIV nef protein in abnormal hematopoiesis in AIDS: in vitro study on bone marrow progenitor cells. Eur J Haematol. 1994;52(2):103–7. doi: 10.1111/j.1600-0609.1994.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 43.Folks TM, Kessler SW, Orenstein JM, Justement JS, Jaffe ES, Fauci AS. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988 Nov 11;242(4880):919–22. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- 44.Neal TF, Holland HK, Baum CM, Villinger F, Ansari AA, Saral R, et al. CD34+ progenitor cells from asymptomatic patients are not a major reservoir for human immunodeficiency virus-1. Blood. 1995 Sep 1;86(5):1749–56. [PubMed] [Google Scholar]
- 45.Varmus H, PT, Heasley S, Simon G, Bishop J. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977;11:307–19. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- 46.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010 Apr;16(4):446–51. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ercoli L, Sarmati L, Parisi SG, Mancino G, De Santis G, Bonanno E, et al. Human immunodeficiency virus infection of human bone marrow stromal myoid cells. Scand J Infect Dis. 1996;28(4):335–40. doi: 10.3109/00365549609037915. [DOI] [PubMed] [Google Scholar]
- 48.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011 Mar;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain research. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2011 Jul 5;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Tang XP, McArthur JC, Scott J, Gartner S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol. 2000 May;6(Suppl 1):S70–81. [PubMed] [Google Scholar]
- 51.Krmpotic-Nemanic J, Vinter I, Kelovizc Z, Marusic A. Postnatal changes of the clivus. Ann Anat. 2005 Jul;187(3):277–80. doi: 10.1016/j.aanat.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG, et al. Bone marrow diffusion measures correlate with dementia severity in HIV patients. AJNR Am J Neuroradiol. 2006 Mar;27(3):589–92. [PMC free article] [PubMed] [Google Scholar]
- 53.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995 Dec;25(12):3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 54.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997 Mar 8;349(9053):692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 55.Koller M, Willheim M, Krugluger W, Kurz M, Hocker P, Forster O, et al. Immunophenotyping of human bone marrow-derived macrophages. Scand J Immunol. 1996 Jun;43(6):626–32. doi: 10.1046/j.1365-3083.1996.d01-265.x. [DOI] [PubMed] [Google Scholar]
- 56.Fagnoni FF, Oliviero B, Zibera C, Gibelli N, Lozza L, Vescovini R, et al. Circulating CD33+ large mononuclear cells contain three distinct populations with phenotype of putative antigen-presenting cells including myeloid dendritic cells and CD14+ monocytes with their CD16+ subset. Cytometry. 2001 Oct 1;45(2):124–32. doi: 10.1002/1097-0320(20011001)45:2<124::aid-cyto1154>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 57.Kim WK, Sun Y, Do H, Autissier P, Halpern EF, Piatak M, Jr., et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010 Apr;87(4):557–67. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001 Dec;7(6):528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 59.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000 Jan;67(1):97–103. [PubMed] [Google Scholar]
- 60.Gartner S. HIV infection and dementia. Science. 2000 Jan 28;287(5453):602–4. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 61.Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984 Mar;65(Pt 3):651–5. doi: 10.1099/0022-1317-65-3-651. [DOI] [PubMed] [Google Scholar]
- 62.Pontisso P, Poon MC, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J (Clin Res Ed) 1984 May 26;288(6430):1563–6. doi: 10.1136/bmj.288.6430.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lie-Injo LE, Balasegaram M, Lopez CG, Herrera AR. Hepatitis B virus DNA in liver and white blood cells of patients with hepatoma. DNA. 1983;2(4):301–8. doi: 10.1089/dna.1983.2.301. [DOI] [PubMed] [Google Scholar]
- 64.Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986 Mar;153(3):471–7. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]
- 65.Blum HE, Stowring L, Figus A, Montgomery CK, Haase AT, Vyas GN. Detection of hepatitis B virus DNA in hepatocytes, bile duct epithelium, and vascular elements by in situ hybridization. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6685–8. doi: 10.1073/pnas.80.21.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romet-Lemonne JL, McLane MF, Elfassi E, Haseltine WA, Azocar J, Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983 Aug 12;221(4611):667–9. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- 67.Laure F, Zagury D, Saimot AG, Gallo RC, Hahn BH, Brechot C. Hepatitis B virus DNA sequences in lymphoid cells from patients with AIDS and AIDS-related complex. Science. 1985 Aug 9;229(4713):561–3. doi: 10.1126/science.2410981. [DOI] [PubMed] [Google Scholar]
- 68.Noonan CA, Yoffe B, Mansell PW, Melnick JL, Hollinger FB. Extrachromosomal sequences of hepatitis B virus DNA in peripheral blood mononuclear cells of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5698–702. doi: 10.1073/pnas.83.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoar DI, Bowen T, Matheson D, Poon MC. Hepatitis B virus DNA is enriched in polymorphonuclear leukocytes. Blood. 1985 Dec;66(6):1251–3. [PubMed] [Google Scholar]
- 70.Michalak TI. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol Rev. 2000 Apr;174:98–111. doi: 10.1034/j.1600-0528.2002.017406.x. [DOI] [PubMed] [Google Scholar]
- 71.Zeldis JB, Dienstag JL, Gale RP. Aplastic anemia and non-A, non-B hepatitis. Am J Med. 1983 Jan;74(1):64–8. doi: 10.1016/0002-9343(83)91119-1. [DOI] [PubMed] [Google Scholar]
- 72.Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986 Aug;78(2):411–7. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeldis JB, Farraye FA, Steinberg HN. In vitro hepatitis B virus suppression of erythropoiesis is dependent on the multiplicity of infection and is reversible with anti-HBs antibodies. Hepatology. 1988 Jul-Aug;8(4):755–9. doi: 10.1002/hep.1840080409. [DOI] [PubMed] [Google Scholar]
- 74.Hwang YY, Liang RH. Hepatitis C in haematological patients. Hepat Res Treat. 2010;2010:961359. doi: 10.1155/2010/961359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006 Apr 15;23(8):1055–65. doi: 10.1111/j.1365-2036.2006.02889.x. [DOI] [PubMed] [Google Scholar]
- 76.Kaushansky K. Thrombopoietin. N Engl J Med. 1998 Sep 10;339(11):746–54. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 77.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002 Nov 15;100(10):3457–69. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 78.Kottilil S, Yan MY, Reitano KN, Zhang X, Lempicki R, Roby G, et al. Human immunodeficiency virus and hepatitis C infections induce distinct immunologic imprints in peripheral mononuclear cells. Hepatology. 2009 Jul;50(1):34–45. doi: 10.1002/hep.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, Chen HY, et al. Effect of viral load on T-lymphocyte failure in patients with chronic hepatitis B. World J Gastroenterol. 2008 Feb 21;14(7):1112–9. doi: 10.3748/wjg.14.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizukoshi E, Sidney J, Livingston B, Ghany M, Hoofnagle JH, Sette A, et al. Cellular immune responses to the hepatitis B virus polymerase. J Immunol. 2004 Nov 1;173(9):5863–71. doi: 10.4049/jimmunol.173.9.5863. [DOI] [PubMed] [Google Scholar]
- 81.Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998 Sep 1;102(5):968–75. doi: 10.1172/JCI3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology. 2001 Apr;33(4):963–71. doi: 10.1053/jhep.2001.23045. [DOI] [PubMed] [Google Scholar]
- 83.Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003 May;23(2):125–36. doi: 10.1055/s-2003-39951. [DOI] [PubMed] [Google Scholar]
- 84.Thio CL, Seaberg EC, Skolasky R, Jr., Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002 Dec 14;360(9349):1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 85.Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005 Mar 24;19(6):593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 86.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010 Jan;85(1):303–15. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991 May;163(5):1138–40. doi: 10.1093/infdis/163.5.1138. [DOI] [PubMed] [Google Scholar]
- 88.Hadler SC, Judson FN, O'Malley PM, Altman NL, Penley K, Buchbinder S, et al. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991 Mar;163(3):454–9. doi: 10.1093/infdis/163.3.454. [DOI] [PubMed] [Google Scholar]
- 89.Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999 Apr;29(4):1306–10. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 90.Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008 Oct 18;22(16):2135–41. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 91.Fang JW, Wright TL, Lau JY. Fibrosing cholestatic hepatitis in patient with HIV and hepatitis B. Lancet. 1993 Nov 6;342(8880):1175. doi: 10.1016/0140-6736(93)92160-u. [DOI] [PubMed] [Google Scholar]
- 92.Guirgis BS, Abbas RO, Azzazy HM. Hepatitis B virus genotyping: current methods and clinical implications. Int J Infect Dis. 2010 Nov;14(11):e941–53. doi: 10.1016/j.ijid.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Martin CM, Welge JA, Blackard JT. Hepatitis B virus (HBV) X gene diversity and evidence of recombination in HBV/HIV co-infected persons. J Med Virol. 2011 Jul;83(7):1142–50. doi: 10.1002/jmv.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Pialoux G, et al. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006 Feb 14;20(3):419–27. doi: 10.1097/01.aids.0000200537.86984.0e. [DOI] [PubMed] [Google Scholar]
- 95.Gunther S, Baginski S, Kissel H, Reinke P, Kruger DH, Will H, et al. Accumulation and persistence of hepatitis B virus core gene deletion mutants in renal transplant patients are associated with end-stage liver disease. Hepatology. 1996 Oct;24(4):751–8. doi: 10.1002/hep.510240401. [DOI] [PubMed] [Google Scholar]
- 96.Preikschat P, Gunther S, Reinhold S, Will H, Budde K, Neumayer HH, et al. Complex HBV populations with mutations in core promoter, C gene, and pre-S region are associated with development of cirrhosis in long-term renal transplant recipients. Hepatology. 2002 Feb;35(2):466–77. doi: 10.1053/jhep.2002.30698. [DOI] [PubMed] [Google Scholar]
- 97.Gunther S, Piwon N, Jung A, Iwanska A, Schmitz H, Will H. Enhanced replication contributes to enrichment of hepatitis B virus with a deletion in the core gene. Virology. 2000 Aug 1;273(2):286–99. doi: 10.1006/viro.2000.0432. [DOI] [PubMed] [Google Scholar]
- 98.Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, et al. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. 2007 Aug 20;21(13):1701–10. doi: 10.1097/QAD.0b013e32826fb305. [DOI] [PubMed] [Google Scholar]
- 99.Thomas DL, Astemborski J, Vlahov D, Strathdee SA, Ray SC, Nelson KE, et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis. 2000 Mar;181(3):844–51. doi: 10.1086/315314. [DOI] [PubMed] [Google Scholar]
- 100.Matthews-Greer JM, Caldito GC, Adley SD, Willis R, Mire AC, Jamison RM, et al. Comparison of hepatitis C viral loads in patients with or without human immunodeficiency virus. Clin Diagn Lab Immunol. 2001 Jul;8(4):690–4. doi: 10.1128/CDLI.8.4.690-694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Zhang T, Ho WZ. Opioids and HIV/HCV infection. J Neuroimmune Pharmacol. 2011 Dec;6(4):477–89. doi: 10.1007/s11481-011-9296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Capa L, Soriano V, Garcia-Samaniego J, Nunez M, Romero M, Cascajero A, et al. Influence of HCV genotype and co-infection with human immunodeficiency virus on CD4(+) and CD8(+) T-cell responses to hepatitis C virus. J Med Virol. 2007 May;79(5):503–10. doi: 10.1002/jmv.20856. [DOI] [PubMed] [Google Scholar]
- 103.Irshad M, Ansari MA, Singh A, Nag P, Raghvendra L, Singh S, et al. HCV-genotypes: a review on their origin, global status, assay system, pathogenecity and response to treatment. Hepatogastroenterology. 2010 Nov-Dec;57(104):1529–38. [PubMed] [Google Scholar]
- 104.Lopez-Labrador FX, Dove L, Hui CK, Phung Y, Kim M, Berenguer M, et al. Trends for genetic variation of Hepatitis C Virus quasispecies in Human Immunodeficiency virus-1 coinfected patients. Virus Res. 2007 Dec;130(1-2):285–91. doi: 10.1016/j.virusres.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sherman KE, Andreatta C, O'Brien J, Gutierrez A, Harris R. Hepatitis C in human immunodeficiency virus-coinfected patients: increased variability in the hypervariable envelope coding domain. Hepatology. 1996 Apr;23(4):688–94. doi: 10.1002/hep.510230405. [DOI] [PubMed] [Google Scholar]
- 106.MacDonald DC, Nelson M, Bower M, Powles T. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era. World J Gastroenterol. 2008 Mar 21;14(11):1657–63. doi: 10.3748/wjg.14.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992-2001. Arch Intern Med. 2004 Nov 22;164(21):2349–54. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 108.McGinnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular carcinoma and non-Hodgkin's lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol. 2006 Nov 1;24(31):5005–9. doi: 10.1200/JCO.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 109.Murillas J, Del Rio M, Riera M, Vaquer P, Salas A, Leyes M, et al. Increased incidence of hepatocellular carcinoma (HCC) in HIV-1 infected patients. Eur J Intern Med. 2005 Apr;16(2):113–5. doi: 10.1016/j.ejim.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 110.McGlynn KA, W.T. L. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15(2):223–43. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Current medical research and opinion. [Evaluation Studies Research Support, Non-U.S. Gov't] 2010 Sep;26(9):2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 112.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011 Aug;54(2):463–71. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahasrabuddhe VV, Shiels MS, McGlynn KA, Engels EA. Hepatobiliary cancers in persons With HIV/AIDS in the United States. Infectious Agents and Cancer. 2012;7(Suppl 1):O25. [Google Scholar]
- 114.Sauleda S, Martorell M, Esteban JI, Tural C, Ruiz I, Puig L, et al. Hepatotoxicity of antiretroviral drugs in HIV HCV patients with congenital coagulopathies followed at an Haemophilia Unit during a decade. Haemophilia. 2006 May;12(3):228–36. doi: 10.1111/j.1365-2516.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- 115.Sterling RK, Orenstein R, Contos MJ, Luketic VA, Sanyal AJ, Mills AS, et al. The spectrum of hepatitis C virus (HCV) in human immunodeficiency virus (HIV) coinfection: Stratification by stage and immune function. Gastroenterology. 2001;120(5):A553. [Google Scholar]
- 116.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2006 Aug 14-28;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 117.Maida I, Soriano V, Castellares C, Ramos B, Sotgiu G, Martin-Carbonero L, et al. Liver fibrosis in HIV-infected patients with chronic hepatitis B extensively exposed to antiretroviral therapy with anti-HBV activity. HIV clinical trials. [Clinical Trial] 2006 Sep-Oct;7(5):246–50. doi: 10.1310/hct0705-246. [DOI] [PubMed] [Google Scholar]
- 118.Mallet VO, Dhalluin-Venier V, Verkarre V, Correas JM, Chaix ML, Viard JP, et al. Reversibility of cirrhosis in HIV/HBV coinfection. Antivir Ther. [Case Reports] 2007;12(2):279–83. [PubMed] [Google Scholar]
- 119.Tuma P, Medrano J, Resino S, Vispo E, Madejon A, Sanchez-Piedra C, et al. Incidence of liver cirrhosis in HIV-infected patients with chronic hepatitis B or C in the era of highly active antiretroviral therapy. Antivir Ther. [Research Support, Non-U.S. Gov't] 2010;15(6):881–6. doi: 10.3851/IMP1630. [DOI] [PubMed] [Google Scholar]
- 120.Macias J, Castellano V, Merchante N, Palacios RB, Mira JA, Saez C, et al. Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS. [Comparative Study Research Support, Non-U.S. Gov't] 2004 Mar 26;18(5):767–74. doi: 10.1097/00002030-200403260-00007. [DOI] [PubMed] [Google Scholar]
- 121.Jones M, Nunez M. HIV and hepatitis C co-infection: the role of HAART in HIV/hepatitis C virus management. Curr Opin HIV AIDS. [Review] 2011 Nov;6(6):546–52. doi: 10.1097/COH.0b013e32834bcbd9. [DOI] [PubMed] [Google Scholar]
- 122.Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001 Aug;34(2):283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 123.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. [Comparative Study Multicenter Study Research Support, N.I.H., Extramural] 2006 Jan;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Tural C, Fuster D, Tor J, Ojanguren I, Sirera G, Ballesteros A, et al. Time on antiretroviral therapy is a protective factor for liver fibrosis in HIV and hepatitis C virus (HCV) co-infected patients. J Viral Hepat. 2003 Mar;10(2):118–25. doi: 10.1046/j.1365-2893.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 125.Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998 Mar 15;83(1-2):77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 126.Arora PK, Fride E, Petitto J, Waggie K, Skolnick P. Morphine-induced immune alterations in vivo. Cell Immunol. 1990 Apr 1;126(2):343–53. doi: 10.1016/0008-8749(90)90326-m. [DOI] [PubMed] [Google Scholar]
- 127.Bryant HU, Bernton EW, Holaday JW. Immunosuppressive effects of chronic morphine treatment in mice. Life Sci. 1987 Oct 5;41(14):1731–8. doi: 10.1016/0024-3205(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 128.Steidl U, Bork S, Schaub S, Selbach O, Seres J, Aivado M, et al. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 2004 Jul 1;104(1):81–8. doi: 10.1182/blood-2004-01-0373. [DOI] [PubMed] [Google Scholar]
- 129.Rozenfeld-Granot G, Toren A, Amariglio N, Nagler A, Rosenthal E, Biniaminov M, et al. MAP kinase activation by mu opioid receptor in cord blood CD34(+)CD38(-) cells. Exp Hematol. 2002 May;30(5):473–80. doi: 10.1016/s0301-472x(02)00786-5. [DOI] [PubMed] [Google Scholar]
- 130.Sei Y, Yoshimoto K, McIntyre T, Skolnick P, Arora PK. Morphine-induced thymic hypoplasia is glucocorticoid-dependent. J Immunol. 1991 Jan 1;146(1):194–8. [PubMed] [Google Scholar]
- 131.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 132.Roy S, Wang J, Charboneau R, Loh HH, Barke RA. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005 Nov 15;175(10):6361–7. doi: 10.4049/jimmunol.175.10.6361. [DOI] [PubMed] [Google Scholar]
- 133.Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006 Sep;1(3):280–95. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 134.Weber JE, Larkin GL, Boe CT, Fras A, Kalaria AS, Maio RF, et al. Effect of cocaine use on bone marrow-mediated erythropoiesis. Acad Emerg Med. 2003 Jul;10(7):705–8. doi: 10.1111/j.1553-2712.2003.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 135.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003 Apr;16(2):209–19. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998 Mar 15;83(1-2):133–8. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 137.Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002 Mar 1;185(5):701–5. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- 138.Rafie C, Campa A, Smith S, Huffman F, Newman F, Baum MK. Cocaine Reduces Thymic Endocrine Function: Another Mechanism for Accelerated HIV Disease Progression. AIDS Res Hum Retroviruses. 2011 Aug;27(8):815–22. doi: 10.1089/aid.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004 Sep;56(3):351–69. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- 140.Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011 Aug;17(4):291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chuang LF, Killam KF, Jr., Chuang RY. Increased replication of simian immunodeficiency virus in CEM x174 cells by morphine sulfate. Biochem Biophys Res Commun. 1993 Sep 30;195(3):1165–73. doi: 10.1006/bbrc.1993.2167. [DOI] [PubMed] [Google Scholar]
- 142.Hao YS, Li PF, Zhang FX, Huang DA, Liu XH, Li G. Morphine alleviates early stages of apoptosis in cultured CEM x174 cells infected with simian immunodeficiency virus. Cell Biol Int. 2003;27(9):719–25. doi: 10.1016/s1065-6995(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 143.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990 Sep;4(9):869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 144.Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, et al. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994 Mar;50(2):167–75. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 145.Bokhari SM, YH, Bethel-Brown C, Fuwang P, Williams R, Dhillon N, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. Journal of NeuroVirology. 2009;15:219–28. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, et al. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004 Oct;78(20):11425–8. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ou DW, Shen ML, Luo YD. Effects of cocaine on the immune system of Balb/C mice. Clin Immunol Immunopathol. 1989 Aug;52(2):305–12. doi: 10.1016/0090-1229(89)90181-5. [DOI] [PubMed] [Google Scholar]
- 148.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002 Jun;26(4):499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 149.Fox L, Alford M, Achim C, Mallory M, Masliah E. Neurodegeneration of somatostatin immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997 Apr;56(4):360–8. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 150.Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001 Apr;60(4):377–85. doi: 10.1093/jnen/60.4.377. [DOI] [PubMed] [Google Scholar]
- 151.Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992 Nov;51(6):585–93. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 152.Kogan M, Rappaport J. HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology. 2011;8:25. doi: 10.1186/1742-4690-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007 Aug;64(16):2120–32. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 155.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008 Jul 11;22(11):1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Front Biosci. 2007;12:1488–95. doi: 10.2741/2162. [DOI] [PubMed] [Google Scholar]
- 157.Zamani S, Radfar R, Nematollahi P, Fadaie R, Meshkati M, Mortazavi S, et al. Prevalence of HIV/HCV/HBV infections and drug-related risk behaviours amongst IDUs recruited through peer-driven sampling in Iran. Int J Drug Policy. 2010 Nov;21(6):493–500. doi: 10.1016/j.drugpo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 158.Bao YP, Liu ZM. Systematic review of HIV and HCV infection among drug users in China. Int J STD AIDS. 2009 Jun;20(6):399–405. doi: 10.1258/ijsa.2008.008362. [DOI] [PubMed] [Google Scholar]
- 159.Mahanta J, Borkakoty B, Das HK, Chelleng PK. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care. 2009 Nov;21(11):1420–4. doi: 10.1080/09540120902862584. [DOI] [PubMed] [Google Scholar]
- 160.Marchesini AM, Pra-Baldi ZP, Mesquita F, Bueno R, Buchalla CM. [Hepatitis B and C among injecting drug users living with HIV in Sao Paulo, Brazil]. Rev Saude Publica. 2007 Dec;41(Suppl 2):57–63. doi: 10.1590/s0034-89102007000900010. [DOI] [PubMed] [Google Scholar]
- 161.Sanvisens A, Fuster D, Serra I, Tor J, Tural C, Rey-Joly C, et al. Estimated liver fibrosis and its impact on all-cause mortality of HCV-monoinfected and HCV/HIV-coinfected drug users. Curr HIV Res. 2011 Jun 1;9(4):256–62. doi: 10.2174/157016211796320298. [DOI] [PubMed] [Google Scholar]
- 162.Graham CS, Wells A, Edwards EM, Herren T, Tumilty S, Stuver SO, et al. Effect of exposure to injection drugs or alcohol on antigen-specific immune responses in HIV and hepatitis C virus coinfection. J Infect Dis. 2007 Mar 15;195(6):847–56. doi: 10.1086/511990. [DOI] [PubMed] [Google Scholar]
- 163.Graham CS, Wells A, Liu T, Sherman KE, Peters M, Chung RT, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. AIDS. 2005 May 20;19(8):767–73. doi: 10.1097/01.aids.0000168970.80551.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]