Abstract
Infertility afflicts fifteen percent of couples who wish to conceive. Despite intensive evaluation of both male and female partners, the etiology may remain unknown leading to a diagnosis of unexplained infertility. For such couples, treatment often entails ovulation induction (OI) with fertility medications coupled with intrauterine insemination. Complications of this therapy include ovarian hyperstimulation syndrome and creation of multiple gestation pregnancies, which can be complicated by preterm labor and delivery, and the associated neonatal morbidity and expense of care for preterm infants. The Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) study is designed to assess whether OI in couples with unexplained infertility with an aromatase inhibitor produces mono-follicular development in most cycles, thereby reducing multiple gestations while maintaining a comparable pregnancy success rate to that achieved by OI with either gonadotropins or clomiphene citrate. These results will provide future guidance of therapy for couples with unexplained infertility, and if comparable pregnancy rates are achieved with a substantial reduction in multiple gestations, the public health benefit will be considerable.
Keywords: Multiple gestation, ovulation induction, gonadotropins, aromatase inhibitor, unexplained infertility
Introduction
Infertility afflicts an estimated 10 to 15% of couples, although this number may underestimate the true incidence because the inability to conceive carries a hidden stigma of shame and secrecy for many couples, and because others cannot afford to seek medical evaluation and treatment.[1, 2, 3] The availability of new treatment methods, including stimulation of ovarian follicular development [commonly called ovulation induction (OI)], intrauterine insemination (IUI) and other assisted reproductive technologies (ART) explain at least in part the observed increase of couples seeking care for infertility (American Society for Reproductive Medicine 1996). Treatment with OI combined with IUI has been applied empirically for different types of infertility including unexplained infertility, male-factor infertility, and anovulatory infertility. This treatment has been shown to be effective in a previous study performed by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network (RMN).[4] Ovulation induction is thought to improve the cycle fecundability rate in part by increasing the number of follicles (and hence oocytes) available for fertilization, and perhaps by correcting subtle, unpredictable ovulatory or endometrial dysfunction. IUI is expected to allow adequate numbers of selected sperm to be deposited into the uterine cavity, thereby circumventing potential cervical problems.
At the present time, primarily two types of medication are used for OI: either an oral selective estrogen receptor modulator, clomiphene citrate (CC), or injectable gonadotropins, each in conjunction with human chorionic gonadotropin (hCG), a surrogate for LH to stimulate ovulation. Administration of these medications, however, is associated with risks for development of ovarian hyperstimulation syndrome (ovarian enlargement, which can be associated with third spacing of fluid, hemoconcentration, and even systemic organ function impairment with the need for hospitalization, sometimes in intensive care units), and initiation of multiple gestation pregnancies, particularly with the use of the more potent gonadotropins. Recently, several descriptive reports have suggested that the use of aromatase inhibitors for ovarian stimulation may be an alternative to CC and gonadotropins, and that such use may be associated with monofollicular development, thereby reducing the rate of multiple gestation pregnancies.[5, 6, 7, 8, 9, 10] The latter is of major concern because of the risk for preterm delivery and its associated fetal morbidity and even mortality, as wsell as the expense of care for multiple infants. Additionally, it has been suggested that use of aromatase inhibitors may be associated with a better endometrial response, including better blood flow as suggested by significantly lower spiral artery impedance on doppler, with the use of the aromatase inhibitor letrozole, as compared to CC.[11] This study will be conducted to assess efficacy of an aromatase inhibitor for ovarian stimulation in women with unexplained infertility.
This manuscript will present the protocol and statistical analysis plan of the RMN study entitled the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial. The RMN studies are designed by its Steering Committee composed of the Principal Investigators of the participating sites and data coordinating center, NICHD Project Scientist, and NICHD appointed Steering Committee Chair. Following development by the Steering Committee, each protocol is reviewed independently by both an Advisory Committee and Data Safety and Monitoring Board (DSMB). Studies undertaken by the RMN are chosen to address critical clinical questions in reproductive science, which require multicenter design to allow timely conduct and completion of the protocol. Access to the clinical data from the AMIGOS trial will be made available to non-RMN investigators for conduct of secondary analyses, as will access to biorepository specimens collected at baseline and throughout the trial, following approval by the RMN Steering Committee. Consequently, knowledge of the design, inclusion criteria, and exclusion criteria of the AMIGOS study are of value to investigators with interest in the reproductive sciences.
Materials and Methods
The AMIGOS study is a multi-center, randomized clinical trial of gonadotropin or clomiphene citrate (CC) vs. letrozole, an aromatase inhibitor (AI), in the treatment of 900 couples with unexplained infertility. While originally designed to be completely double blinded including sham injections in women assigned to the CC or AI arms, inability to identify a financially feasible placebo to the gonadotropin injection resulted in a final study design in which the study will be double-blind with respect to CC and AI use only. An equal number of patients will be randomized to receive gonadotropins, CC, or AI daily according to computer-generated randomization schedule. Treatment assignments will be stratified by site and age (18-34 and 35-40). The randomization scheme will be a stratified randomization with permuted blocking (the block size is permuted between 3 and 6 in the ratio of 2:1) within each stratum. Subjects will receive one of three medications in the form of a pill (over coated CC or over coated AI in double-blinded fashion) or gonadotropin injection.
Study candidates will be recruited from the clinics of the Reproductive Medicine Network and affiliated entities after obtaining written informed consent from the women and their partners. Recruited subjects will meet the inclusion and exclusion criteria detailed below. Monitoring of this trial at all sites will be conducted by the RMN Data Coordination Center, with progress reports provided to the RMN Data and Safety Monitoring Board no less than once every three months in order to review trial progress and subject safety. The Food and Drug Administration has issued IND# 107705 for the use of an aromatase inhibitor in ovarian stimulation.
The overall goal of the inclusion/exclusion criteria is to identify a population of healthy infertile women who are regularly ovulating. These will include a normal uterine cavity with at least one patent tube and a partner with motile sperm count of at least 5 million in the ejaculate.
The female partner will undergo a physical examination within 60 days prior to randomization, and instructed to take prenatal vitamins or folic acid. All patients and partners will complete a risk factor questionnaire for genetic diseases; if appropriate, each site will provide a referral for counseling. Baseline measurements to be conducted within six months prior to initiation of cycle 1 therapy are: height (without shoes), body weight, and abdominal circumference (at the umbilicus). Additional baseline samples which will be obtained during cycle days 1-10 preceding initiation of therapy are: 1) fasting glucose and insulin levels, 2) Serum testosterone, SHBG, DHEAS level, and day 3 ± 1 E2, LH, FSH, prolactin, TSH, anti-mullerian hormone, Inhibin A and B, and antral follicle count. In addition to subject demographics, validated assessment will be conducted for the subject and their partner for quality of life, (Medical Outcome Survey, Short Form (SF-36)[12], Medical Outcome Survey (Prime MD-PHQ)[12] FertiQol Survey), sleep patterns (Sleep Habits questionnaire)[13], and sexual function [Female Sexual Distress Scale (FSDS)[14] and Female Sexual Function Inventory (FSFI)[14] (for females), and International Index of Erectile Function (IIEF)[15] (for males)], and quality of life with infertility FertiQol Survey.[16]
Additional baseline characteristics for the male partner to be assessed include semen analysis, semen (sperm) for microarray analysis, semen specimen (for DNA, RNA, micro RNA, and protein), testosterone, SHBG, LH, FSH, Inhibin B, insulin, glucose and TSH.
Ovarian stimulation will be conducted in the usual clinical manner, commencing with cycle day 3 monitoring of serum estradiol and transvaginal ultrasound exam for measurement of ovarian and follicular size, and follicular number. Medication as determined by the randomization arm will be begun on cycle day 3 and continued for five days in the CC and aromatase inhibitor arms; with monitoring of serum estradiol and follicular size and number periodically until criteria for HCG administration are met. In the gonadotropin arm, women will initially receive four days of therapy with subsequent dosage determined by serum estradiol and transvaginal ultrasound monitoring. HCG administration (10,000 IUI) criteria will be the same for all study arms, with intrauterine insemination (IUI) performed within 40 hours after HCG administration.
Results
It is difficult to identify estimates of pregnancy and multiple gestation rates in infertile couples with a regularly ovulating female receiving empiric ovulation induction. For the determination of sample size calculations for the AMIGOS study, the RMN has utilized a recent report of a cohort study of women who underwent 3045 cycles of ovulation induction using a variety of agents for OI, including gonadotropins alone, CC alone, and AI alone5 in which choice of treatment agent was based on the decision of the patient and her health care provider. The study evaluated pregnancy outcomes and multiple gestation rates among 3045 cycles contributed by 1650 infertile patients. Pregnancy occurred in 110 of 671 cycles (16.4%) in which gonadotropins alone were administered, which is within the rates previously reported for gonadotropins in this category of women (see Table 1). Among women receiving clomiphene citrate, pregnancy occurred in 80 of 994 cycles (8%), which is also within the range described in the literature (see Table 1). With the AI, letrozole at a dose of 2.5 mg/d for five days, pregnancy occurred in 33 of 167 cycles (19.8%); while at a dose of 5.0 mg/d for five days, 70 of 432 (16.2%) cycles resulted in conception.[5] With such pregnancy rates, the cumulative pregnancy rate after four cycles of ovulation induction could be estimated to range from approximately 48% with per cycle rates of 15%, to a 34% cumulative pregnancy rate if per cycle rates were 10%; these estimated rates are within the cumulative pregnancy rates previously reported.[4,5, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] We have powered this study conservatively assuming a cumulative pregnancy rate of 30% in the gonadotropins treatment arm after up to four cycles of therapy, which is consistent with the rate achieved during the previous prospective randomized RMN clinical trial on superovulation and insemination.[4] In order to achieve a reduction in multiple birth rates, we decided we are willing to accept a reduction of the cumulative pregnancy rate with AI (as opposed to gonadotropins) of up to 25% (thus, a cumulative pregnancy rate of 22.5%), but for purposes of this study, we have conducted power calculations utilizing the conservative assumption that cumulative pregnancy rate will be 10% less in the AI group.
Table 1.
AMIGOS Trial Pregnancy and Multiple Gestation Rate Power Table
| N (each arm) | Test for Gonadotropins-CC Combined vs. AI |
Pregnancy Rate Test – Non-inferiority Score test |
|---|---|---|
| 167 | .79 | 0.39 |
| 180 | .81 | 0.41 |
| 200 | .83 | 0.44 |
| 220 | .84 | 0.46 |
| 230 | .85 | 0.48 |
| 240 | .86 | 0.49 |
| 250 | .87 | 0.51 |
| 260 | .88 | 0.52 |
| 270 | .90 | 0.53 |
In the cohort study described above, multiple gestation rates were approximately 11% in women receiving gonadotropins, 15% in women receiving CC, and 5% in each of the letrozole groups.[5] This rate for multiple gestations with gonadotropins is lower than the 29% multiple rate in the prior report by the RMN following treatment with gonadotropins plus intrauterine inseminations5 (as will be conducted in this protocol). Collectively, these are the basis for the 25% multiple gestation rate that we have assumed for our sample size and power calculations. The multiple gestation rate for the CC group in the cohort study was 15%, is the basis for the 12.5% rate we have assumed. These multiple gestation rates form the basis for our hypothesis of a 75% and 50% reduction in multiple pregnancy rates with letrozole ovarian stimulation compared to gonadotropins or CC, respectively. For the AMIGOS trial, fetal heartbeat identified by early first trimester ultrasound will be used as the primary endpoint of multiple gestations; this will allow us to avoid errors due to spontaneous miscarriages or selective fetal reductions later in gestation. Secondary outcomes will include the overall pregnancy rate, as well as the multiple gestational pregnancy live birth rate, which will reflect a reduction in the multiple rates not only from selective fetal reduction, but also spontaneous gestational loss of some, or all, fetuses.
Statistics
To assess multiple gestation rate differences among the three treatment groups we will first test the combined gonadotropin and CC groups vs. the AI group using a one-sided z test at the .05 significance level. We will also do the pair wise comparisons of each of the gonadatropin and CC groups with the AI group. The proportions achieving clinical pregnancies will be calculated on an intent-to-treat basis.
For the secondary outcome of pregnancy rate, the non-inferiority test will compare the differences among the overall pregnancy rates in the three groups using a 25% reduction in the letrozole population pregnancy rate compared to the combined set of the gonadotropin & CC groups as a basis for the null-hypothesis, a value (δ0=0.25) that represents the maximum value acceptable as “clinically unimportant” (see power section below). Further analyses for the pregnancy outcome will make use of logistic regression which will allow us to include, in addition to the dummy coded variables representing treatment, those patient and medical variables expected to be associated with becoming pregnant. Such patient characteristics would include factors such as age, day 3 serum FSH level, duration of infertility, BMI, history of previous live birth and history of prior ovarian surgery. We will also include in the Logistic Regression analysis selected interaction terms for those factors that may be related to treatment differences e.g. maternal age. Evaluation of differential treatment effects due to patient or other medical factors should lead to better estimates of treatment effects by taking into account other factors affecting the likelihood of becoming pregnant.
Treatment effects for a number of tertiary outcomes will also be assessed. These include: 1) average number of ovarian follicles developed per cycle, 2) hormone levels, 3) endometrial thickness or change development, 4) number of adverse events including ovarian hyperstimulation syndrome, 5) length of treatment cycle, and 6) cost of treatment. To compare these outcomes among the treatment groups, chi square tests will be used for categorical variables. For continuous outcomes which are approximately normally distributed, t tests will be used. Mann-Whitney tests will be used for ordinal or highly skewed dependent variables. Within-treatment-group correlations will also be used to investigate the relations of these outcomes to relevant patient characteristics. To evaluate effects of treatment on number of cycles needed to achieve pregnancy we will use Kaplan-Meier curves evaluated by the log-rank test. If the assumption of proportional within-group rates over time (cycles) holds, the addition use of Cox regression analysis will allow us to evaluate additional medical or patient factors related to this outcome as well as possible interactions of these factors with treatment.
Power
To assess the power for the primary outcome of multiple births we are conservatively assuming 30%, 27%, and 27% pregnancy rates for the gonadotropin, CC and AI populations, respectively, and a 25% pregnancy loss for each group. In the power analysis we assume the rates of multiple pregnancies will be 25% in the gonadotropin group, 12.5% in the CC group, and 6.25% in the AI group. The primary evaluation of power is based on a comparison of the combined gonadatropin and CC groups (n=144) vs. the AI group (n=72) using a one-sided z test with a significance level of .05. Under the above assumptions power for this test is .94. We will also test the pair wise comparisons of gonadatropin vs. AI (power =.94) and CC vs. AI (power =.39). We note that if the CC population rate is actually higher, e.g.15 (a quite plausible value from the literature) then the power for the AI comparison would be .56. An equally plausible alternative value for the AI proportion might be .04 in which case power for the CC comparison would be p=.60. All the above power estimates come from the Cohen tables based on arc sine transformations of the proportions.
We will also perform a non-inferiority analysis among the gonadotropin, CC and letrozole treatments with respect to the secondary outcomes of pregnancy and live birth rates. The power for the non-inferiority test for group differences in pregnancy rates assumes 25% reduction in the AI population pregnancy rate compared to the combination of the gonadotropin and CC groups’ rates (28.5%). This value is the margin (δ0 = 0.25) that represents the maximum acceptable reduction as “clinically unimportant”. Under H0 δ, the difference between the combination of the gonadotropin & CC populations’ rates (28.5%) and the AI population rate (27%), is δ0; under H1 δ < δ0. If we have 240 subjects in the AI group and 480 subjects in the combined gonadotropin & CC group and a one-sided alpha of 0.05, power is approximately 49%. It should be pointed out that when we do the comparisons among the three groups we will adjust for various other predictive factors, e.g., site and age, which may reduce the error term. Consequently, these power figures may be conservative.
An interim analysis is planned at the mid-point of the study to assess the overall pregnancy rate (i.e., the three groups combined) while maintaining blinding of treatment assignment in order to assess whether this pregnancy rate approximates the predicted rates, and whether additional subjects may be needed to meet power targets. Since treatment assignments will not be identified, it is not thought that adjustments in significance levels will be required.
Discussion
The occurrence of multiple gestational pregnancy is one of the most common problems associated with OI. While the rate of spontaneous twin pregnancy has been estimated to range from 1%–1.35% and that of a triplet pregnancy from 0.01%–0.017%, the incidence of multiple gestational pregnancies is more than 10-20 times higher with OI and IUI, ranging from 7.5%– 29% per couple.[18,19] In the last decade, the significant increase in the incidence of multiple births in most countries is almost entirely attributable to the use of gonadotropins and other pharmaceutical agents for OI.[25] The magnitude of this problem is exemplified by the report of the National Vital Statistics Reports for 2001, in which the rate of twinning has increased 33% since 1990 and 59% since 1980, while the rate of triplets and higher-order multiples has increased more than 400% since 1980.[26] Multiple gestational pregnancies resulting from IVF and related treatments such as oocyte and embryo donation may be prevented prior to conception by limiting the number of embryos transferred and increasing the use of single embryo transfer. These practices are starting to slow the occurrence of high order multiple gestational pregnancies resulting from IVF. Nonetheless, treatment with OI and IUI account for more than half of the multiple gestational pregnancies that occur with ART, and currently preconceptual methods have not been identified that maintain effective pregnancy rates while preventing multiple gestational pregnancies.
The major concern with multiple gestations is the high rate of preterm delivery and its associated consequences. In 2001, 1.6% of singletons, 11.8% of twins, 36.7% of triplets, 64.5% of quadruplets, and 78.6% of quintuplets were delivered before 32 completed weeks of gestation.27 Even more noteworthy, the incidence of extreme prematurity (delivery before 28 weeks) for both triplets and quadruplets may be as high as 14%.[27,28,29] The increased incidence of maternal and neonatal complications associated with multiple gestational pregnancies has been well documented,[30, 31, 32, 33, 34, 35] and the greater likelihood of prematurity alone contributes substantially to neonatal risk.[36, 37, 38] Additionally, hospital costs for each twin or triplet infant can be twice or three times that of a singleton, and lifetime costs to the healthcare system and community may be 100 to 200 times that of a singleton.[39,40] After controlling for variables known to affect hospital charges, the predicted total charges to the family in 1991 for a singleton delivery were $9,845, as compared with $37,947 for twins ($18,974 per baby) and $109,765 for triplets ($36,588 per baby).[39] Notwithstanding these facts, currently there is substantial economic pressure and demand from patients for infertility treatment programs to increase their success rates.
One approach to reduce the incidence of multiple gestations from OI with gonadotropins is to avoid the use of gonadotropins in non-ART cycles and proceed directly to offering ART in couples who have failed to conceive with clomiphene citrate and intrauterine inseminations. Using this approach in the Fast Track and Standard Treatment Trial (FASTT) Reindollar and colleagues[41] were unable to demonstrate a benefit of gonadotropin/IUI therapy as an intermediary step in the progression from clomiphene citrate/IUI to ART therapy. However, this study was conducted in Massachusetts which has far more state mandated insurance coverage for ART than most states in the USA. Thus, use of ART may not be a financially viable alternative for many couples wishing to conceive.
Although often viewed as a blessing by some longstanding infertile couples who now have “two for the price of one,” the considerable human and financial costs of multiple births warrant a re-evaluation of the protocols of OI. Obviously, the best strategy to reduce multiple births is preventing the occurrence of multiple gestational pregnancy, rather than dealing with the problem after its occurrence (e.g., selective multi-fetal pregnancy reduction). Selective multi-fetal reduction presents an ethical problem for many couples, entails the risk of losing all fetuses being carried, and increases psychological stress for couples who have struggled through years of infertility.[42, 43, 44, 45] An ovulation induction agent that would achieve ovarian mono-follicular development in most cycles, thereby reducing multiple gestations (and possibly other complications such as ovarian hyperstimulation syndrome), with a comparable pregnancy success rate to OI with gonadotropins or CC, would be an exciting advance for enhancing the opportunity for parenthood while ameliorating (or at least substantially reducing) the burden of multiple gestational pregnancies following OI with IUI.
Whether our hypothesis of the value of aromatase inhibitors for ovulation induction in couples with unexplained infertility is confirmed or not, the results of the NIH/NICHD Cooperative Reproductive Medicine Network AMIGOS Trial is expected to have significant impact on future clinical management of couples with unexplained infertility who desire to conceive. The AMIGOS Trial will examine whether the anecdotal experience of low multiple gestation rates with the use of letrozole for OI in descriptive, non-randomized studies will persist in a randomized prospective study design in couples with unexplained infertility. Additionally, the presumably lower rate of ovarian hyperstimulation syndrome associated with OI with letrozole will be evaluated. Importantly though, it will assess whether these outcomes are achievable without a detrimental effect on the overall conception and live birth rates resulting from OI with letrozole compared to gonadotropin or clomiphene citrate therapy.
Ovarian hyperstimulation is a potentially life threatening condition in which young, previously healthy women can require intensive care in a matter of days. The syndrome is thought to result from leakage of fluid from mesothelial tissues surfaces and the ovaries into the abdominal (and pleural and pericardial) cavities, with intravascular hemoconcentration. Symptoms, including pelvic pain and pressure, are positively correlated with the number of follicles which develop and the degree of elevation of the serum estradiol level. (However, the same markers of elevated estradiol and number of follicles, is generally also associated with increased occurrence of conception). Accumulation of ascites can be associated with a wide variety of complications including shortness of breath (due to limited diaphragmatic excursions) impaired renal function, venous thrombosis, and disseminated intravascular coagulation. There are no treatments for ovarian hyperstimulation syndrome, although supportive measures have included paracentesis, consumption of high protein diet, and symptomatic treatments. Ovarian hyperstimulation syndrome is usually selflimited and begins to resolve after seven to ten days if pregnancy does not ensue. However, in women who conceive, secretion of HCG from trophoblastic tissue can worsen and prolong symptoms.
Even if the pregnancy and live birth rates following OI with an aromatase inhibitor are inferior to those achievable with gonadotropins or clomiphene citrate in couples with unexplained infertility, if the multiple gestational pregnancy rates are reduced, OI treatment with AI may become the treatment of choice. Additionally, this study will examine the conception and live birth rates, as well as the rate of OHSS, when employing a moderate dose gonadotropin protocol, with defined criteria for both administering and withholding HCG to induce the final stages of oocyte maturation and follicle rupture. Thus it will be possible to assess which factors affect the undesirable outcomes (multiple births and OHSS) including gonadotropic use per se, gonadotropin dosage or adherence to HCG administration guidelines.
Conclusion
The occurrence of multiple gestations after ovulation induction in couples with unexplained infertility remains a major concern of infertility therapy. The AMIGOS study will evaluate whether the use of an aromatase inhibitor for recruitment of multiple follicular development will lower the rate of multiple pregnancy without detrimentally affecting the likelihood of establishing a pregnancy.
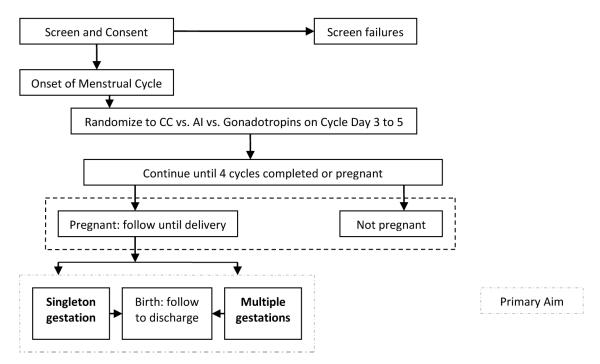
Figure 1. AMIGOS Trial Flowchart.
The flowchart above summarizes this study the NICHD Cooperative Reproductive Medicine Network with our central hypothesis, that use of aromatase inhibitors will stimulate the ovaries sufficiently to produce no reduction in the rate of pregnancy, while significantly reducing the numbers of multiple gestational pregnancies as compared to stimulation with CC or gonadotropins.
Acknowledgement
The authors express their thanks to the other members of the Cooperative Reproductive Medicine Network (RMN), as well as the RMN Advisory Committee and Data Safety Monitoring Board for their critical review of the AMIGOS protocol.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5U10 HD 039005, 3U10 HD 039005-S1, 5U10 HD 038992, 3U10 HD 038992-S1, 5U10 HD 055942, 3U10 HD 055942-S1, 5U10 HD 055944, 3U10 HD 055944-S1, 5U10HD055925, 5U10HD055925-S1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schroeder P. Infertility and the world outside. Fertil Steril. 1988;49(5):765–7. doi: 10.1016/s0015-0282(16)59880-x. [DOI] [PubMed] [Google Scholar]
- [2].Downey J, Yingling S, McKinney M, Husami N, Jewelewicz R, Maidman J. Mood disorders, psychiatric symptoms, and distress in women presenting for infertility evaluation. Fertil Steril. 1989;52(3):425–32. doi: 10.1016/s0015-0282(16)60912-3. [DOI] [PubMed] [Google Scholar]
- [3].Whiteford LM, Gonzalez L. Stigma: the hidden burden of infertility. Soc Sci Med. 1995;40(1):27–36. doi: 10.1016/0277-9536(94)00124-c. [DOI] [PubMed] [Google Scholar]
- [4].Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med. 1999;340(3):177–83. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- [5].Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol. 2005;192(2):381–6. doi: 10.1016/j.ajog.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [6].Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;18(8):1588–97. doi: 10.1093/humrep/deg311. [DOI] [PubMed] [Google Scholar]
- [7].Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22(1):61–78. doi: 10.1055/s-2004-823028. [DOI] [PubMed] [Google Scholar]
- [8].Al-Fozan H, Al-Khadouri M, et al. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82(6):1561–63. doi: 10.1016/j.fertnstert.2004.04.070. [DOI] [PubMed] [Google Scholar]
- [9].Al-Fadhli R, Sylvestre C, et al. A randomized trial of superovulation with two different doses of letrozole. Fertil Steril. 2006;85(1):161–65. doi: 10.1016/j.fertnstert.2005.07.1283. [DOI] [PubMed] [Google Scholar]
- [10].Bayer U, Tanriverdi HA, et al. Letrozole vs. Clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85(4):1045–48. doi: 10.1016/j.fertnstert.2005.09.045. [DOI] [PubMed] [Google Scholar]
- [11].Baruah J, Roy KK, Rahman SM, Kumar S, Sharma JB, Karmakar D. Endometrial effects of letrozole and clomiphene citrate in women with polycystic ovary syndrome using spiral artery Doppler. Arch Gynecol Obstet. 2009;279:311–14. doi: 10.1007/s00404-008-0714-4. [DOI] [PubMed] [Google Scholar]
- [12].Ware JE, SK, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. New England Medical Center, The Health Institute; Boston: 1993. [Google Scholar]
- [13].Kump K, Whalen C, Tishler PV, Browner I, Ferrette V, Strohl KP, et al. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respiratory and Critical Care Medicine. 1994;150:735–41. doi: 10.1164/ajrccm.150.3.8087345. [DOI] [PubMed] [Google Scholar]
- [14].Rosen R, Brown C, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- [15].Rosen R, Riley A, Wagner G, et al. The international Index of Erectile Dysfunction (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 49:822–30. doi: 10.1016/s0090-4295(97)00238-0. 197. [DOI] [PubMed] [Google Scholar]
- [16].FertiQoL FertiQoL was developed by Jacky Boivin, Janet Takefman and Andrea Braverman with sponsorship from the European Society of Human Reproduction & Embryology (ESHRE), American Society for Reproductive Medicine (ASRM) and Merck-Serono S.A. ESHRE and ASRM jointly hold the rights to FertiQoL. www.mcgillsymposium.com/FertiQoL/fertiqol_international.asp.
- [17].Melis GB, Paoletti AM, et al. Pharmacologic induction of multiple follicular development improves the success rate of artificial insemination with husband’s semen in couples with male-related or unexplained infertility. Fertil Steril. 1987;47(3):441–445. doi: 10.1016/s0015-0282(16)59052-9. [DOI] [PubMed] [Google Scholar]
- [18].Shelden R, Kemmann E, Bohrer M, Pasquale S. Multiple gestation is associated with the use of high sperm numbers in the intrauterine insemination specimen in women undergoing gonadotropin stimulation. Fertil Steril. 1988;49(4):607–10. doi: 10.1016/s0015-0282(16)59826-4. [DOI] [PubMed] [Google Scholar]
- [19].Valbuena D, Simon C, Romero JL, Remohi J, Pellicer A. Factors responsible for multiple pregnancies after ovarian stimulation and intrauterine insemination with gonadotropins. J Assist Reprod Genet. 1996;13(8):663–8. doi: 10.1007/BF02069646. [DOI] [PubMed] [Google Scholar]
- [20].Serhal PF, Katz M, et al. Unexplained infertility – the value of Pergonal superovulation combined with intrauterine insemination. Fertil Steril. 1988;49(4):602–606. doi: 10.1016/s0015-0282(16)59825-2. [DOI] [PubMed] [Google Scholar]
- [21].Hughes EG. The effectiveness of ovulation induction and intrauterine insemination in the treatment of persistent infertility: a meta-analysis. Hum Reprod. 1997;12(9):1865–1872. doi: 10.1093/humrep/12.9.1865. [DOI] [PubMed] [Google Scholar]
- [22].Tadokoro N, Vollenhoven B, et al. Cumulative pregnancy rates in couples with anovulatory infertility compared with unexplained infertility in an ovulation induction programme. Hum Reprod. 1997;12(9):1939–1944. doi: 10.1093/humrep/12.9.1939. [DOI] [PubMed] [Google Scholar]
- [23].Cohlen BJ, te Velde ER, et al. Controlled ovarian hyperstimulation and intrauterine insemination for treating male subfertility: a controlled study. Hum Reprod. 1998;13(6):1553–1558. doi: 10.1093/humrep/13.6.1553. [DOI] [PubMed] [Google Scholar]
- [24].Gleicher N, Oleske DM, et al. Reducing the risk of high-order multiple pregnancy after ovarian stimulation with gonadotropins. N Engl J Med. 2000;343(1):2–7. doi: 10.1056/NEJM200007063430101. [DOI] [PubMed] [Google Scholar]
- [25].Dawood MY. In vitro fertilization, gamete intrafallopian transfer, and superovulation with intrauterine insemination: efficacy and potential health hazards on babies delivered. Am J Obstet Gynecol. 1996;174(4):1208–17. doi: 10.1016/s0002-9378(96)70663-4. [DOI] [PubMed] [Google Scholar]
- [26].National Vital Statistics Reports Births: final data for 2001. p. 51. [PubMed]
- [27].Collins MS, Bleyl JA. Seventy-one quadruplet pregnancies: management and outcome. Am J Obstet Gynecol. 1990;162(6):1384–91. doi: 10.1016/0002-9378(90)90896-f. discussion 1391-2. [DOI] [PubMed] [Google Scholar]
- [28].Kaufman GE, Malone FD, Harvey-Wilkes KB, Chelmow D, Penzias AS, D’Alton ME. Neonatal morbidity and mortality associated with triplet pregnancy. Obstet Gynecol. 1998;91(3):342–8. doi: 10.1016/s0029-7844(97)00686-8. [DOI] [PubMed] [Google Scholar]
- [29].Devine PC, Malone FD, Athanassiou A, Harvey-Wilkes K, D’Alton ME. Maternal and neonatal outcome of 100 consecutive triplet pregnancies. Am J Perinatol. 2001;18(4):225–35. doi: 10.1055/s-2001-15505. [DOI] [PubMed] [Google Scholar]
- [30].De Muylder X, Moutquin JM, Desgranges MF, Leduc B, Lazaro-Lopez F. Obstetrical profile of twin pregnancies: a retrospective review of 11 years (1969-1979) at Hospital Notre-Dame, Montreal, Canada. Acta Genet Med Gemelol (Roma) 1982;31(3-4):149–55. doi: 10.1017/s0001566000008230. [DOI] [PubMed] [Google Scholar]
- [31].Blotting BJ, Davies IM, Macfarlane AJ. Recent trends in the incidence of multiple births and associated mortality. Arch Dis Child. 1987;62(9):941–50. doi: 10.1136/adc.62.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lipitz S, Reichman B, Paret G, Modan M, Shalev J, Serr DM, et al. The improving outcome of triplet pregnancies. Am J Obstet Gynecol. 1989;161(5):1279–84. doi: 10.1016/0002-9378(89)90683-2. [DOI] [PubMed] [Google Scholar]
- [33].Lipitz S, Frenkel Y, Watts C, Ben-Fafael Z, Barkai G, Reichman B. High-order multifetal gestation-management and outcome. Obstet Gynecol. 1990;76(2):215–8. [PubMed] [Google Scholar]
- [34].Levene MI, Wild J, Steer P. Higher multiple births and the modern management fo infertility in Britain. The British Association of Perinatal Medicine. Br J Obstet Gynaecol. 1992;99(7):607–13. doi: 10.1111/j.1471-0528.1992.tb13831.x. [DOI] [PubMed] [Google Scholar]
- [35].Jain T, Missmer SA, Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. N Engl J Med. 2004;350(16):1639–45. doi: 10.1056/NEJMsa032073. [DOI] [PubMed] [Google Scholar]
- [36].Newman RB, Hamer C, Miller MC. Outpatient triplet management: a contemporary review. Am J Obstet Gynecol. 1989;161(3):547–53. doi: 10.1016/0002-9378(89)90354-2. discussion 553-5. [DOI] [PubMed] [Google Scholar]
- [37].Elster AD, Bleyl JL, Craven TE. Birth weight standards for triplets under modern obstetric care in the United States, 1984-1989. Obstet Gynecol. 1991;7793:387–93. [PubMed] [Google Scholar]
- [38].Kiely JL, Kleinman JC, Kiely M. Triplets and higher-order multiple births. Time trends and infant mortality. Am J Dis Child. 1992;146(7):862–8. doi: 10.1001/archpedi.1992.02160190094029. [DOI] [PubMed] [Google Scholar]
- [39].Callahan TL, Hall Je, Ettner SL, Christiansen CL, Greene MF, Crowley WF., Jr The economic impact of multiple-gestation pregnancies and the contribution of assisted-reproduction techniques to their incidence. N Engl J Med. 1994;331(4):244–9. doi: 10.1056/NEJM199407283310407. [DOI] [PubMed] [Google Scholar]
- [40].Bergh T, Ericson A, Hillensjö T, Nygren KG, Wennerholm UB. Deliveries and children born after in-vitro fertilization in Sweden 1982-95: a retrospective cohort study. Lancet. 1999;354(9190):1579–85. doi: 10.1016/S0140-6736(99)04345-7. [DOI] [PubMed] [Google Scholar]
- [41].Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94(3):888–99. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- [42].Howie PW. Selective reduction in multiple pregnancy. Bmj. 1988;297(6646):433–4. doi: 10.1136/bmj.297.6646.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wapner RJ, Davis GH, Johnson A, Weinblatt VJ, Fischer RL, Jackson LG, et al. Selective reduction of multifetal pregnancies. Lancet. 1990;335(8681):90–3. doi: 10.1016/0140-6736(90)90550-o. [DOI] [PubMed] [Google Scholar]
- [44].Berkowitz RL, Lynch L, Lapinski R, Bergh P. First-trimester transabdominal multifetal pregnancy reduction: a report of two hundred completed cases. Am J Obstet Gynecol. 1993;169(1):17–21. doi: 10.1016/0002-9378(93)90124-2. [DOI] [PubMed] [Google Scholar]
- [45].Braude P, Rowell P. Assisted conception. III-problems with assisted conception. Bmj. 2003;327(7420):920–3. doi: 10.1136/bmj.327.7420.920. [DOI] [PMC free article] [PubMed] [Google Scholar]