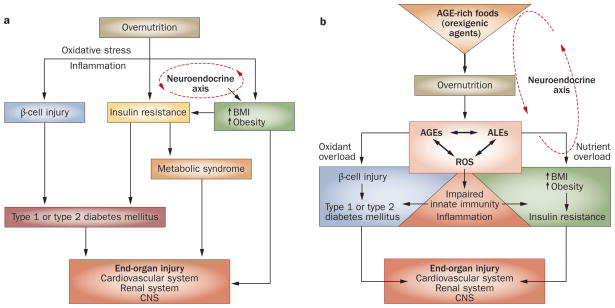
Figure 1.
Detrimental effects of overnutrition. a | The classic scheme: high levels of pro-oxidant AGEs result from chronic hyperglycemia and contribute to diabetic complications. Overnutrition promotes inflammation and insulin resistance. Excess nutrient availability (via neuroendocrine networks) stimulates food intake, which promotes weight gain and obesity. These conditions are associated with increased inflammation, known to underlie insulin sensitivity, β-cell injury and diabetes mellitus. Chronic hyperglycemia increases intracellular oxidative stress (that is, from mitochondria or the endoplasmic reticulum) and increases the risk of complications, including cardiovascular disease, retinopathy, chronic kidney disease and neuropathy, involving the central and peripheral nervous systems. b | A paradigm shift: AGEs precede diabetes mellitus. Modern food contains appetite-enhancing AGEs, which promote food consumption and overnutrition, leading to increased BMI, obesity and diabetes mellitus, as well as oxidant overload. Sustained influx of nutrient AGEs and ALEs leads to suppression of innate host defenses and a surplus of intracellular ROS, which increases the basal oxidant stress and inflammation. The combination of these processes can simultaneously cause β-cell dysfunction, impaired insulin secretion and insulin resistance, as well as diabetic complications. Restriction of food-derived AGEs reduces oxidative stress and prevents or improves type 1 and type 2 diabetes mellitus in mice. Abbreviations: AGE, advanced glycation endproduct; ALE, advanced lipoxidation endproduct; CNS, central nervous system; ROS, reactive oxygen species.