Abstract
Survivors of critical illness frequently have profound and long-lasting cognitive impairments and psychiatric disorders, which adversely affect functional outcomes including return to work, and quality of life. While data regarding cognitive outcomes has been increasing over the last 15 years, neuroimaging data in medical and surgical critical populations is extremely limited. The abrupt development of new significant cognitive impairments after critical illness along with abnormalities on neuroimaging suggest that critical illness results in new acquired brain injury, similar to that observed in other acquired brain injuries. Abnormalities on neuroimaging including cortical and subcortical lesions, brain atrophy, and WMHs which occur in widely distributed brain regions. Given the high prevalence rate of cognitive impairments in this population, neuroimaging is important to help elucidate neuropathology of critical illness acquired brain injury. Research is needed across imaging modalities in order to fully understand the effect of critical illness on the brain and to help understand the effects of rehabilitation, including cognitive rehabilitation in survivors of critical illness.
Keywords: critical illness, cognitive impairment, neuroimaging, ICU, lesions
Introduction
Advancement in the treatment of critical illness has resulted in reduced mortality over the last 30 years resulting in millions of patients who survive a critical illness (Adhikari, Fowler, Bhagwanjee, & Rubenfeld, 2010). Outcomes research over last 15 years has demonstrated that many intensive care unit (ICU) survivors develop significant encephalopathy and neurologic morbidities, including neuromuscular morbidities (central and peripheral), cognitive impairments, and psychiatric disorders such as depression, anxiety and posttraumatic stress disorder. Cognitive outcomes have been studied in a variety of critically ill populations including medical/surgical, sepsis, can acute respiratory distress syndrome, with high rates of cognitive impairment reported across populations. Among mechanically ventilated general medical ICU survivors approximately 2 out of every 3 patients will develop moderate to severe cognitive impairments (Hopkins & Jackson, 2009). Among the sickest ICU patients, such as patients with acute respiratory distress syndrome (ARDS), the prevalence of long-term cognitive impairments is particularly high; 74% of patients at hospital discharge in one study, with 46% of ARDS patients impaired at 1 and 2 years.(Hopkins, Weaver, et al., 2005a) Further, the cognitive impairments in ICU survivors are often new and develop during or after the onset of critical illness as two recent large population based studies indicate (Ehlenbach et al., 2010; Iwashyna, Ely, Smith, & Langa, 2010).
The etiology and mechanisms of post-ICU cognitive impairments are unclear. To date only a few studies have assessed mechanisms of cognitive impairment in critically ill populations. Pathophysiologic mechanisms of cognitive impairment identified to date include hypoxemia (Hopkins, Weaver, et al., 2005a), hypotension (Hopkins, Weaver, Chan, & Orme, 2004), glucose dysregulation (Hopkins et al., 2010), and inflammation and cytokine activated immune system dysregulation (Elenkov, Iezzoni, Daly, Harris, & Chrousos, 2005). It is likely that these and other mechanisms work in concert with individual risk factors such as genetic markers, age, and co-morbid chronic medical disorders in the development of cognitive impairments.
The abrupt development of new significant cognitive impairments after critical illness likely reflects the existence of an acquired brain injury (Hopkins & Jackson, 2006), an idea supported by neuroimaging findings (Hopkins, Gale, & Weaver, 2006; Suchyta, Jephson, & Hopkins, 2010). This paper will review neuroimaging data in critically ill populations and will discuss the implications of critical illness acquired brain injury for neurorehabilitation.
Structural Neuroimaging
Widely-distributed abnormalities on neuroimaging are common following brain injuries such as hypoxia, stroke, and trauma and these abnormalities are often associated with adverse cognitive outcomes (Lerner & Rosenstein, 2000). Unlike other neurologic disorders, such as traumatic brain injury or dementia, there is very limited neuroimaging data in critically ill populations. However, investigations of neurologic outcome using neuroimaging modalities in ICU populations are increasing. Early neuroimaging studies failed to find brain abnormalities in medical ICU populations. For example a study of 12 patients with sepsis found no abnormalities or lesions in these patients (A. C. Jackson, Gilbert, Young, & Bolton, 1985). A computed tomography (CT) neuroimaging study in 69 critically ill patients, only 4 patients underwent imaging, none of whom had structural abnormalities (Young et al., 1990). More recently CT and MR studies suggest that critically ill patients develop abnormalities on structural brain imaging. These discrepant findings may be due to the fact that more recent imaging and quantitative image analysis techniques are increasingly sophisticated and sensitive in detecting brain abnormalities.
In mixed medical/surgical population of 235 critically ill patients (80 patients with trauma) admitted to a medical ICU who underwent neuroimaging, 83 (35%) patients had structural abnormalities including 21 patients who had subarachnoid hemorrhage, 11 patients had subdural hemorrhage, and 29 patients had widely distributed lesions in both hemispheres.(Kishi, Iwasaki, Takezawa, Kurosawa, & Endo, 1995) Twenty-one patients had multiple structural abnormalities. Not surprisingly trauma patients had a higher rate of lesions compared to non-trauma patients, 44% vs. 31% respectively. The trauma patients had higher rates of subdural (n = 10) and subarachnoid (n = 18) hemorrhage compared to non-trauma patients (3 patients had subarachnoid hemorrhages and 1 patient had a subdural hemorrhage). Structural abnormalities on brain imaging were not associated with increased risk of developing delirium in these patients. The abnormalities occurred early in the ICU course, in many cases on the day of ICU admission, suggesting the abnormities were new due to pathophysiological changes (Kishi et al., 1995).
Lesions are reported in both cortical and subcortical regions. Finelli and Uphoff (2004) report a case of a 48 years old female with sepsis who underwent brain imaging. Brain MR imaging on Day 4 showed lesions in basal ganglia (bilateral), cerebellum, brain stem, and temporal lobe regions (Finelli & Uphoff, 2004). These brain regions are at the end of the vascular supply and are sensitive to the effects of hypoxia (Hopkins, Tate, & Bigler, 2005). The patient subsequently died and post-mortum examination showed extensive bilateral basal gangila damage in the caudate nucleus, putamen and globus pallidus bilaterally and a small infarctions were reported in the cortex and splenium of the corpus callosum. Microorganisms were not found in the brain, suggestion the lesiosnwere not due to brain infection. The hippocampus, a medial temporal lobe structure important for memory had mild neuronal loss and acute neruonal ischemic changes. There brain stem and cerebellum had wide spread edema (Finelli & Uphoff, 2004).
A larger retrospective cohort study assessed structural changes on brain imaging and cognitive outcomes in 64 critically ill patients who developed neurological changes (e.g. diminished level including loss of consciousness, confusion, altered mental status, coma, or prolonged delirium) during their intensive care unit (ICU) stay (Suchyta et al., 2010). Cognitive impairments including memory deficits and executive dysfunction occurred in 22 (48%) of the survivors. Abnormalities on brain CT and MR imaging occurred in 41 (64.1%) of ICU patients who had neurologic changes. Abnormalities included 26 patients with atrophy, 19 with white matter hyperintensities (WMHs), 17 with lesions, 7 with focal hemorrhages, and 4 with encephalomalacia. Lesion location was heterogeneous and included 7 patients with lesions in the frontal lobes, 6 patients with lesions in the parietal lobes, 3 patients with lesions in the temporal lobes, and one patient had an occipital lobe lesion. The WMHs were widely distributed throughout the brain, 15 patients had periventricular and 10 patients had centrum semiovale WMHs. While many lesions appear to occur early in the course of critical illness, they can manifest throughout the course of the illness. Seven patients’ initial brain scans were normal, but structural abnormalities were present on a later scan including WMHs, hemorrhage, and atrophy. The presence of multiple chronic medical disorders did not predict structural abnormalities and there were no associations between brain abnormalities and age, ARDS, premorbid substance abuse, premorbid psychiatric disorders, hypoxemia, hypotension, steroids, or sedative use (Suchyta et al., 2010).
A small quantitative brain imaging study in 15 of 66 critically ill patients with ARDS assessed structural lesions in these patients by clinical radiological CT reports (Hopkins et al., 2006). Of the 15 patients, seven patients had no abnormalities on brain imaging. Eight patients had abnormalities on brain imaging including lesions in the pons and parietal lobe, small petechial hemorrhagic lesion, diffuse atrophy, enlargement of the ventricles, sulcal widening, and hippocampal atrophy (Hopkins et al., 2006).
A recent prospective case series in eight ICU survivors with delirium who underwent brain MR imaging as part of clinical care found 6 (75%) patients had WMHs and one patient had mild generalized brain atrophy (Morandi et al., 2010). White matter hyperintensities occurred in cortical and subcortical regions. Not surprisingly younger patients had smaller what matter lesions than older patients. No patients had hemorrhagic lesions on neuroimaging. All six patients with WMHs and/or brain atrophy had severe cognitive impairments; including impaired memory, executive function, and attention. These findings indicate that critical illness may result in new brain injury shown by structural abnormalities on neuroimaging and significant cognitive impairments (Morandi et al., 2010).
Table 1 shows structural brain imaging findings in ICU patients. While the studies are few in number, structural imaging studies to date indicate abnormalities on brain imaging range from 35% to75% of patients. The lesions occurred in diffuse brain regions including cortical and subcortical structures (e.g. basal ganglia, hippocampus, corpus callosum), atrophy, white matter lesions, and hemorrhagic lesions (Kishi et al., 1995; Morandi et al., 2010; Suchyta et al., 2010). Taken altogether, these findings provide evidence that critical illness can result in new acquired brain injury manifest by structural abnormalities on brain imaging.
Table 1.
Abnormalities on structural brain imaging.
| Study | n | Percent of patients with abnormalities | Structural Lesions | WMHs | Hemorrhagic Lesions | Atrophy |
|---|---|---|---|---|---|---|
| Case Studies | ||||||
| Jackson et al. 2009 (J. C. Jackson, Hopkins, et al., 2009) | 1 | 100% | No | No | No | Yes |
| Holling et al., 2000 (Hollinger et al., 2000) | 1 | 100% | No | Yes | No | No |
| Finelli et al., 2004 (Finelli & Uphoff, 2004) | 1 | 100% | Yes | Yes | Yes | No |
| Piazza et al., 2009 (Piazza et al., 2009) | 4 | 50% | No | Yes | No | No |
| Group Studies | ||||||
| Kishi et al. 1995 (Kishi et al., 1995) | 235 | 35% | Yes | Yes | Yes | Yes |
| Suchyta et al. 2010 (Suchyta et al., 2010) | 64 | 64.1% | Yes | Yes | Yes | Yes |
| Morandi et al. 2010 (Morandi et al., 2010) | 8 | 75% | No | Yes | No | Yes |
| Hopkins et al. 2006 (Hopkins et al., 2006) | 15 | 53.3% | Yes | Yes | Yes | Yes |
Quantitative Neuroimaging
Several studies have measured brain volumes to assess atrophic changes in survivors of critical illness. A recent case study described a 49 year old female with sepsis and ARDS with no history of neurologic or psychiatric disorders who underwent neuropsychological assessments and brain magnetic resonance (MR) imaging at ICU discharge and approximately 3.5 years post-ICU discharge (J. C. Jackson, Hopkins, et al., 2009). Brain MR imaging at 3.5 years showed marked atrophy (her brain volume was more than 8 standard deviations smaller than matched controls) with sulcal widening and ventricular enlargement (1.3 SD larger than controls) that were not present at hospital discharge. The patient had significant decline in intellectual function (a decrease in test scores of ~2 SD) and had impaired memory, attention and executive function. The cognitive impairments mirrored the brain atrophy, supporting the idea that critical illness results in the development of new brain injury (J. C. Jackson, Hopkins, et al., 2009).
As described above, a small quantitative brain imaging study in 15 of 66 critically ill patients with ARDS found ventricular enlargement and larger ventricle-to-brain (VBR) ratios in these patients along with sulcal widening and an increase in cerebral spinal fluid (Hopkins et al., 2006). Brain imaging in an ARDS patients shows imaging at approximately hospital discharge and one and two years; Figure 1 shows brain MR scans in an ARDS patient who had ventricular enlargement and Figure 2 shows atrophy of the corpus callosum in the same patients. The atrophy occurred between hospital discharge and one and two years post-ICU discharge. The patients also had increased temporal horn volumes, suggesting temporal lobe atrophy (Hopkins et al., 2006). Following traumatic brain injury an increase in temporal horn volumes is associated with hippocampal atrophy (Bigler et al., 1997) or loss of temporal lobe white matter (Bigler, Anderson, & Blatter, 2002). The brain atrophy was not associated with illness severity or duration and severity of hypoxemia, likely due to the small sample size or variability in atrophy (i.e. ventricle volumes) (Hopkins et al., 2006). One limitation of this and most neuroimaging studies in ICU populations is the lack of pre-ICU imaging and only a few studies have obtained imaging early in the ICU course due to the nature of critical illness (e.g. physiologic instability, mechanical ventilation, high doses of sedative medications, etc.). Imaging early in the ICU course may provide an ICU baseline by which later scans can be compared to determine if there are changes over time, as Figures 1 and 2 illustrate. Thus, neuroimaging early in the ICU stay can help delineate the extent and severity of brain injury and help to rule-out pre-morbid structural abnormalities.
Figure 1.
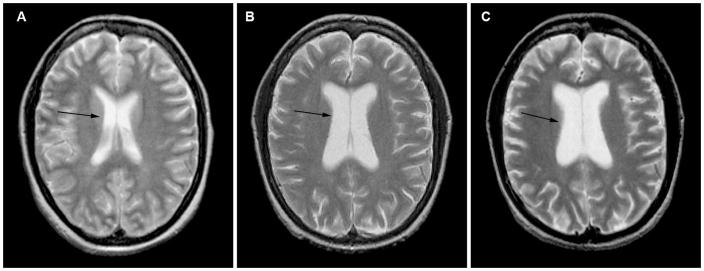
Figure 2 shows an axial magnetic resonance (MR) scans through the body of the lateral ventricles in a patient with acute respiratory distress syndromes at three different time points. The arrows point to the body of the lateral ventricles. Figure A shows a baseline axial MR scan obtained just prior to hospital discharge showing normal lateral ventricles. Figure B shows an axial MR scan at one year post-hospital discharge showing significant enlargement of the lateral ventricles in the same patient. Figure C shows a mid-sagittal MR scan at two years showing enlargement of the lateral ventricles which have increased in size from one to two years, indicating some additional atrophy.
Figure 2.
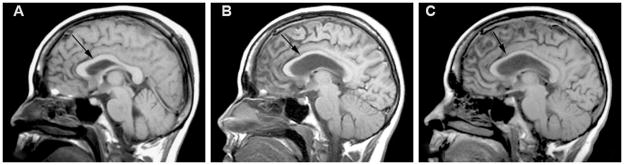
Figure 2 shows mid-sagittal magnetic resonance (MR) scans through the corpus callosum in the same patient with acute respiratory distress syndromes at three different time points. The arrows point to the corpus callosum. Figure A shows a baseline mid-sagittal MR scan obtained just prior to hospital discharge showing a normal corpus callosum (no atrophy). Figure B shows a mid-sagittal MR scan at one year post-hospital discharge showing significant corpus callosum atrophy. Figure C shows a mid-sagittal MR scan at two years showing atrophy of the corpus callosum, which appears unchanged from the one year scan.
A prospective study in 47 critically ill patients assessed the relationship between delirium duration, cognitive function, and quantitative brain volumes. A longer duration of delirium during ICU treatment was associated with greater brain atrophy (VBR) at hospital discharge and 3 month follow-up (Gunther ML, EPub Ahead of Print) Greater brain atrophy at 3 months was associated with worse cognitive function at 12 months. Smaller cerebellar, thalamic, and anterior cingulate volumes were found at 3 months which were associated with impaired executive function and attention at 12 months (Gunther ML, EPub Ahead of Print). The findings of significant brain atrophy and associated cognitive impairments are similar to findings in patients following anoxic brain injury and traumatic brain injury (Hopkins, Tate, et al., 2005; Johnson, 1994), suggesting a relationship between brain atrophy and adverse cognitive outcome in these critically ill patients. Further, delirium may be a potential mechanism for brain atrophy and cognitive impairments in this population. Additional studies are needed to evaluate the effects of critical illness on brain atrophy and to assess potential mechanisms of the observed atrophy.
Neuroimaging of White Matter
As described previously WMHs are common neuroimaging findings in survivors of critical illness (Hopkins et al., 2006; Morandi et al., 2010; Suchyta et al., 2010). White-matter hyperintensities are bright areas that are observed in white matter regions on a varitiey of MR imaging sequences including T2-weighted or mixed-weighted images, fluid attenuated inversion (FLAIR) sequences and more recently diffusion tensor imaging (DTI). Subsets of critically ill patients develop severe infections (i.e. sepsis or septic shock) that are associated with adverse neurologic outcomes. A single 49 year old male with sepsis underwent serial MR imaging (Hollinger, Zurcher, Schroth, & Mattle, 2000). Computed tomography of the identified several areas of hypo-dense white matter bilaterally on the second ICU day. Imaging at one week found WMHs that were enhanced with contrast (larger in size). At 3 and 4 weeks white matter lesions appeared to be diminishing but remained present (Hollinger et al., 2000).
Piazza and colleagues used MR imaging and measured cerebral spinal fluid S100B to assess neuronal injury in four patients, two patients had sepsis and two patients developed septic shock (Piazza, Cotena, De Robertis, Caranci, & Tufano, 2009). Two of the patients had WMHs, one in the cortical and subcortical frontal regions and the other had periventricular WMHs. Interestingly, the two pateints with normal brain imaging had abnormal electroencephlogram findings and elevated S100B levels, a marker of neuronal damage and blood brain barrier disruption (Piazza et al., 2009). Given the very small sample size in this study, it is difficult to draw firm conclusions, but the data suggest that not only does sepsis result in abnormalties on brain imaging but it appears to damage the brain even in the absence of neuroimaging findings.
White matter lesions have been reported on MRI and neuropathological studies in patients with sepsis (Sharshar et al., 2007). A study in 9 patients with sepsis found 5 patients had centrum semiovale and deep white matter WMHs, two patients had ischemic changes post-stroke, and 2 patients had normal brain imaging. Further, the size of the WMHs increased over time. One patient subsequently died and a post-mortem examination found white matter edema and dilation of the perivascular spaces. The white matter lesions were associated with an increased length of shock and worse outcome at 90 days, measured by the Glasgow Outcome Scale (Sharshar et al., 2007). The above findings suggest that patients with sepsis or septic shock are at high risk for brain injury manifest by abnormalities on brain imaging, predominately in the white matter. The white matter lesions appear to be associated with adverse functional outcomes, although this finding needs to be replicated.
Newer imaging techniques such as diffusion tensor imaging (DTI) are also sensitive to white matter injury (Xu, Rasmussen, Lagopoulos, & Haberg, 2007) and allows assessment of white matter integrity (Little et al., 2010). White matter integrity is measured using fractional anisotrophy (FA) and lower FA values (values range from 0 to 1) indicate axonal injury. While WMHs have been demonstrated in critically ill patients, questions remain regarding their functional implications. A recent study in critically ill patients used DTI to assess the relationship between delirium, white matter integrity, and cognitive function (Morandi A, 2012). Fifty general medical and general surgical critically ill patients with delirium underwent neuroimaging. A longer duration of delirium was associated with white matter injury (lower FA values) in the corpus callosum and anterior limb of the internal capsule at hospital discharge and 3 months. White matter injury was associated with cognitive impairments at 3 and 12 months (Morandi A, 2012). The identification of white matter injury using FA provides additional evidence that critical illness can and does injure the brain and such injury is associated with adverse cognitive outcomes in these patients.
The clinical significance and prognostic value of the WMHs are the subject of discussion and require further research. White matter hyperintensities appear to be associated with cognitive impairments, especially larger lesions, however any lesion if located in a critical cognitive pathway, can have significant adverse effects on cognitive function. Research is needed to understand the etiotlogie and functional significance of WMHs in critically ill populations.
Spectroscopy
There are no studies in humans that have used spectroscopy to assess neurological outcomes in critically ill patients. A study in septic mice used proton spectroscopy to assess neuronal metabolism by measuring the relative amounts of choline (Ch) a marker of neuronal death, total creatine (Cr) a marker of mitochondrial dysfunction, and N-acetylasparate (NAA) a marked of neuronal damage (Bozza et al., 2010). Proton spectra were acquired in the hippocampus and a strong decrease in the NAA/Ch ratio was found, indicating neuronal damage in septic mice. Research is needed to determine if similar findings occur in humans with sepsis (Bozza et al., 2010).
Amyloid Photon Emission Tomography
There are no studies in critically ill populations which have utilized Amyloid photon emission tomography (PET) imaging. However, the use of amyloid imaging agents such as Florebetapir have been heralded as one of the major advances in the study of mild cognitive impairment and dementia in the last decade. A recent editorial in the Journal of the American Medical Association (JAMA) heralded Amyloid PET for its’ “crucial role in establishing the relevance of brain β-amyloid burden in the development and progression of neurodegenerative disorders including Alzheimer’s disease (Breteler, 2011). Amyloid PET imaging may be particularly relevant in the study of survivors of critical illness as numerous studies suggest that inflammatory processes (prominent in critical illness) contribute to the accumulation of amyloid, believed to be a central and crucial step in the emergence of Alzheimer’s disease (Guo, Yu, Grass, de Beer, & Kindy, 2002; Mhatre, Floyd, & Hensley, 2004; Senthilkumar, Chang, & Jayakumar, 2008).
Implications for Neurorehabilitation
As the above data indicate, critical illness is associated with brain injury manifest by lesions and atrophy on brain imaging and cognitive impairments; further brain injury may be a vehicle thru which dementia develops. Cognitive impairments often persist years after ICU discharge (J. C. Jackson, Mitchell, & Hopkins, 2009) and as such, research is needed to prevent or ameliorate these difficulties. Until such a time as preventive steps are identified, however, the focus should be on rehabilitation and remediation, as is done with more traditional brain injured populations (i.e. anoxic and traumatic brain injuries). There is a large gap between the high rate of cognitive morbidity following critical illness and referral for cognitive rehabilitation. Unfortunately, few critically ill survivors are referred for cognitive rehabilitation and those who are predominately are referred for physical rehabilitation (Hopkins, Weaver, et al., 2005b). The literature regarding cognitive rehabilitation is sparse. Two small randomized controlled trials assessed the effect of physical rehabilitation on functional outcomes assessed using the Functional Independence Measurement score, including the cognitive domain score as a secondary outcome (Chen et al., 2011; Chiang, Wang, Wu, Wu, & Wu, 2006). Both studies showed improved cognitive function in the physical rehabilitation treatment group compared to the control group.
The effect of cognitive rehabilitation in ICU populations is just beginning to be studied, though it may be a fruitful avenue for future study. Only one study to date, the RETURN Trial (J. C. Jackson et al., 2012), has assessed the effect of cognitive rehabilitation, specifically focusing on rehabilitation of executive function, one of the most common cognitive impairments observed in ICU survivors. Medical ICU patients were randomized to either a program of cognitive and physical rehabilitation for 12 weeks using two-way video teleconference and in-person visits or usual care. At 3-month follow-up the intervention group patients demonstrated significantly improved executive functioning (J. C. Jackson et al., 2012). Research on cognitive rehabilitation of ICU survivors is in its infancy and studies are needed to determine the effects of cognitive rehabilitation on a wide range of cognitive deficits that have been observed in ICU populations.
Conclusions
Brain injury following critical illness has been defined by neuropsychological performance to date. Neuroimaging is important to help elucidate neuropathology and brain function given the high prevalence rate of neurologic and cognitive impairments in this population. Understanding neurological compromise in the critical care patient may be optimized by obtaining baseline neuroimaging, done early in the hospital course, followed by imaging at later time points that allow for the assessment of change over time, if present. Questions remain regarding the effect of critical illness on brain structures and function. These questions can be most fully explored via neuroimaging, including functional MR imaging, as is recognized by increasing numbers of critical care researchers who are using imaging modalities. Examining neuroimaging findings in ICU patients may help us understand the post-ICU long-term neurologic and cognitive morbidities and prognosis for ICU survivors. Further neuroimaging research is necessary to fully understand the effects of critical illness on the brain, and to help understand the effects of rehabilitation, including cognitive rehabilitation in these patients.
Footnotes
Conflicts of Interest: None
References
- Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23(2):255–266. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18(1):11–23. [PMC free article] [PubMed] [Google Scholar]
- Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, Castro-Faria-Neto HC. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30(2):440–448. doi: 10.1038/jcbfm.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM. Mapping out biomarkers for Alzheimer disease. JAMA. 2011;305(3):304–305. doi: 10.1001/jama.2010.2017. [DOI] [PubMed] [Google Scholar]
- Chen SY, Su CL, Wu YT, Wang LY, Wu CP, Wu HD, Chiang LL. Physical training is beneficial to functional status and survival in patients with prolonged mechanical ventilation. Journal of the Formosan Medical Association. 2011;110(9):572–579. doi: 10.1016/j.jfma.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86(9):1271–1281. doi: 10.2522/ptj.20050036. [DOI] [PubMed] [Google Scholar]
- Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJPA, Carson SS, Curtis JR, Larson EB. Association Between Acute Care and Critical Illness Hospitalization and Cognitive Function in Older Adults. Jama-Journal of the American Medical Association. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Finelli PF, Uphoff DF. Magnetic resonance imaging abnormalities with septic encephalopathy. Journal of Neurology Neurosurgery and Psychiatry. 2004;75(8):1189–1191. doi: 10.1136/jnnp.2003.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther ML, MA, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, III, Canonico A, Merkle K, Cannistraci CJ, Rogers BP, Gatenby JC, Heckers S, Gore JC, Hopkins RO, Ely EW. The association between brain volumes, delirium duration and cognitive outcomes in intensive care unit survivors: A prospective cohort magnetic resonance imaging study: The VISIONS prospective cohort magnetic resonance imaging study. Critical Care Medicine. doi: 10.1097/CCM.0b013e318250acc0. EPub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JT, Yu J, Grass D, de Beer FC, Kindy MS. Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci. 2002;22(14):5900–5909. doi: 10.1523/JNEUROSCI.22-14-05900.2002. 20026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger P, Zurcher R, Schroth G, Mattle HP. Diffusion magnetic resonance imaging findings in cerebritis and brain abscesses in a patient with septic encephalopathy. J Neurol. 2000;247(3):232–234. doi: 10.1007/s004150050573. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of Acute Respiratory Distress Syndrome. Brain Inj. 2006;20(3):263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130(3):869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Jackson JC. Short- and long-term cognitive outcomes in intensive care unit survivors. Clin Chest Med. 2009;30(1):143–153. ix. doi: 10.1016/j.ccm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Suchyta MR, Snow GL, Jephson A, Weaver LK, Orme JF. Blood glucose dysregulation and cognitive outcome in ARDS survivors. Brain Inj. 2010;24(12):1478–1484. doi: 10.3109/02699052.2010.506861. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Tate DF, Bigler ED. Anoxic versus traumatic brain injury: amount of tissue loss, not etiology, alters cognitive and emotional function. [Comparative Study] Neuropsychology. 2005;19(2):233–242. doi: 10.1037/0894-4105.19.2.233. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Chan KJ, Orme JF., Jr Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10(7):1005–1017. doi: 10.1017/s135561770410711x. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2005a;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005b;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. Jama-Journal of the American Medical Association. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Gilbert JJ, Young GB, Bolton CF. The encephalopathy of sepsis. Can J Neurol Sci. 1985;12(4):303–307. doi: 10.1017/s0317167100035381. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Ely EW, Morey MC, Anderson VM, Denne LB, Clune J, Hoenig H. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med. 2012;40(4):1088–1097. doi: 10.1097/CCM.0b013e3182373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Hopkins RO, Miller RR, Gordon SM, Wheeler AP, Ely EW. Acute respiratory distress syndrome, sepsis, and cognitive decline: a review and case study. South Med J. 2009;102(11):1150–1157. doi: 10.1097/SMJ.0b013e3181b6a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009;25(3):615–628. x. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Johnson SCB, Erin D, Burr, Robert B, Blatter, Duane D. White matter atrophy, ventricular dilation, and intellectual functioning following traumatic brain injury. Neuropsychology. 1994;8(3):307–315. [Google Scholar]
- Kishi Y, Iwasaki Y, Takezawa K, Kurosawa H, Endo S. Delirium in Critical Care Unit Patients Admitted through an Emergency Room. General Hospital Psychiatry. 1995;17(5):371–379. doi: 10.1016/0163-8343(95)00056-w. [DOI] [PubMed] [Google Scholar]
- Lerner DM, Rosenstein DL. Neuroimaging in delirium and related conditions. [Review] Semin Clin Neuropsychiatry. 2000;5(2):98–112. doi: 10.153/SCNP00500098. [DOI] [PubMed] [Google Scholar]
- Little DM, Kraus MF, Jiam C, Moynihan M, Siroko M, Schulze E, Geary EK. Neuroimaging of hypoxic-ischemic brain injury. NeuroRehabilitation. 2010;26(1):15–25. doi: 10.3233/NRE-2010-0532. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis. 2004;6(2):147–157. doi: 10.3233/jad-2004-6206. [DOI] [PubMed] [Google Scholar]
- Morandi A, Gunther ML, Vasilevskis EE, Girard TD, Hopkins RO, Jackson JC, Ely EW. Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont) 2010;7(9):28–33. [PMC free article] [PubMed] [Google Scholar]
- Morandi ARB, Gunther ML, Merkle K, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, 3rd, Canonico A, Cannistraci CJ, Gore JC, Ely EW, Hopkins RO. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study. Critical Care Medicine. 2012 doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. [Case Reports] Neurochem Res. 2009;34(7):1289–1292. doi: 10.1007/s11064-008-9907-2. [DOI] [PubMed] [Google Scholar]
- Senthilkumar S, Chang E, Jayakumar R. Diffusible amyloid oligomers trigger systemic amyloidosis in mice. Biochem J. 2008;415(2):207–215. doi: 10.1042/BJ20071696. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, Annane D. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33(5):798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- Suchyta MR, Jephson A, Hopkins RO. Neurologic changes during critical illness: brain imaging findings and neurobehavioral outcomes. Brain Imaging Behav. 2010;4(1):22–34. doi: 10.1007/s11682-009-9082-3. [DOI] [PubMed] [Google Scholar]
- Xu J, Rasmussen IA, Lagopoulos J, Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. Journal of Neurotrauma. 2007;24(5):753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with septic illness. Clin Invest Med. 1990;13(6):297–304. [PubMed] [Google Scholar]


