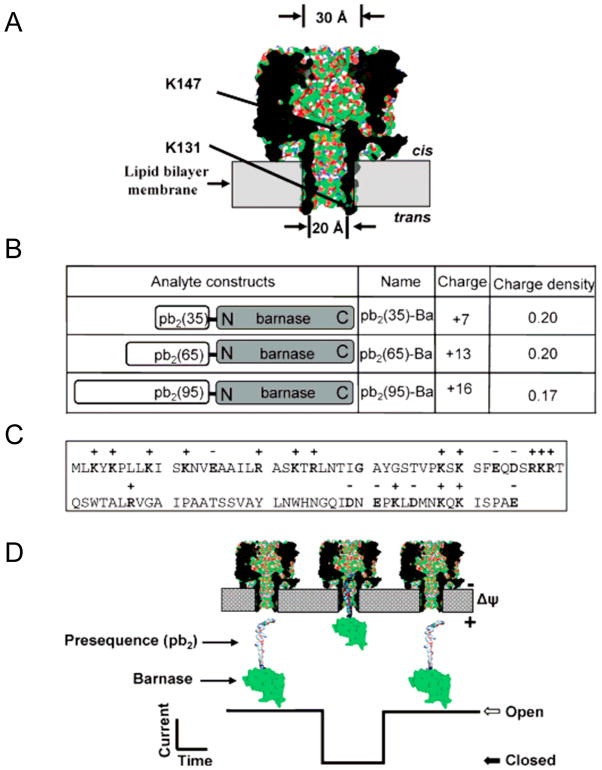
Fig. 1.
Engineered αHL protein pores and pb2-Ba proteins for protein sensing: (A) Strategic functionalization of the αHL protein pores. Two mutations that introduced two electrostatic traps (acidic rings of aspartates) are engineered at either the trans opening (K131) or the cis opening (K147) of the β barrel, as shown by arrows. PyMOL53 was used to generate the view using the coordinates from the crystal structure of the αHL pore (7ahl.pdb); (B) Molecular design of the analyte proteins (pb2-Ba). Three different lengths of Yeast pre-cytochrome b2 (pb2) with different charge densities were fused to the N-terminus of barnase (Ba); (C) The panel shows the amino acid sequence of the first 95 residues of the pb2 presequence; (D) Illustration of how pb2-Ba protein partitions into the αHL protein pore from the trans side of the bilayer. This single-molecule partitioning is monitored by a transient blockade in the single-channel electrical trace. Figure was reproduced, with permission, from Ref (27).