Fig 2.
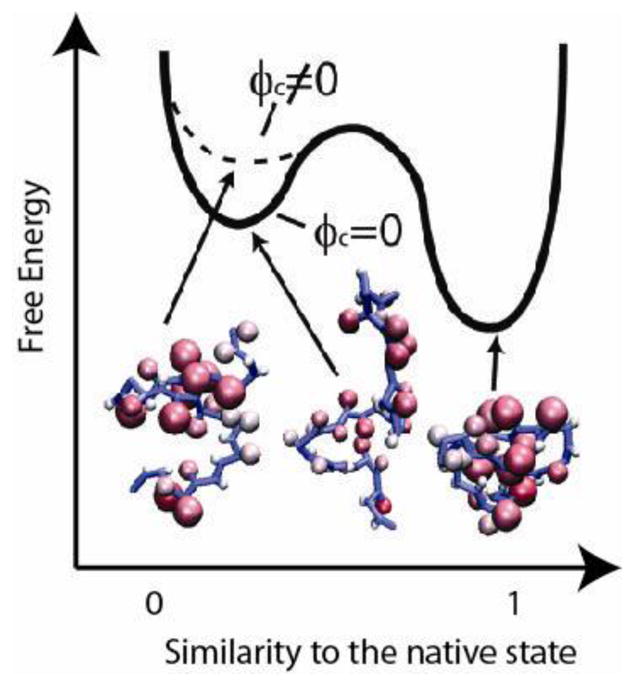
Fig 2[20]. To illustrate the macromolecular crowding effect on the folding energy landscape, we plot a schematic diagram of the free energy against an order parameter, such as the fraction of native contact formation Q. When Q is close to l, the corresponding basin represents the folded state; when Q is close to 0, the corresponding basin represents the unfolded state. Upon the addition of hard-core crowders, the ensemble of unfolded states become compact and the free energy of the unfolded states are destabilized. As a result, the folded state of a protein is relatively stabilized. This shift in free energy is entropically driven.
