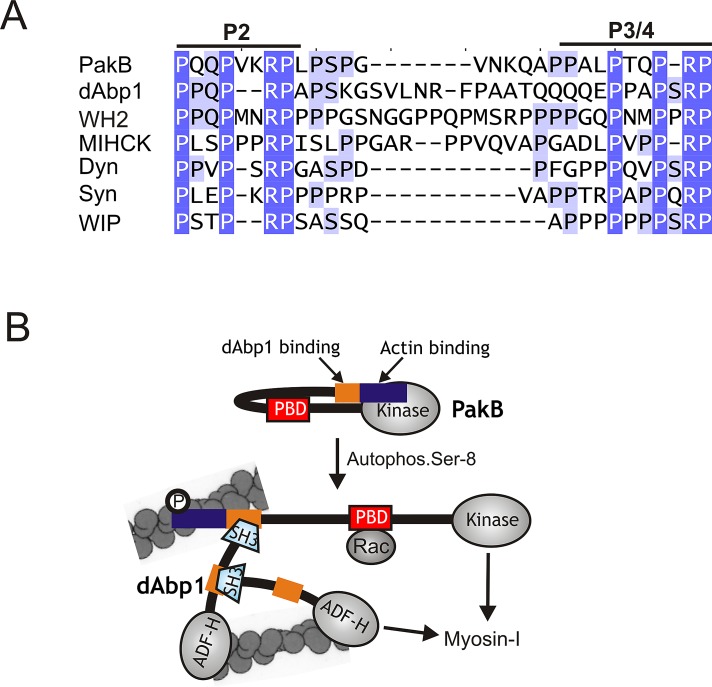
FIGURE 8:
PakB interacts with the dAbp1 SH3 domain via a conserved proline-rich sequence. (A) Sequences similar to the two binding sites for the dAbp1 SH3 domain in PakB (P2 and P3/4; residues 64–91) are conserved in dAbp1 (residues 265–297), a Dictyostelium WH2 domain–containing protein (WH2; residues 361-396), Acanthamoeba myosin I heavy chain kinase (MIHCK; residues 207–239), human dynamin-1 (Dyn; residues 813–836); human synaptojanin-1 (Syn; residues 1141–1165), and human WAS/WASL-interacting protein family member 1 (WIP-1; residues 295–316). GenBank accession numbers are as follows: WH2 domain-containing protein (EAL66623), MIHCK (AAD09141), dynamin-1 (AAH50279), synaptojanin-1 (NP_003886), and WIP-1 (NP_003378). (B) A model for the PakB–dAbp1 complex. Inactive PakB is believed to adopt a folded conformation in which the N-terminal region suppresses the activity of the PBD and kinase domains. Autophosphorylation of Ser-8, which occurs in the absence of allosteric effectors, allows GTP-Rac to bind the PBD domain (red box) and increases kinase activity 40-fold. It is proposed that Ser-8 autophosphorylation causes PakB to unfold, which would increase exposure of the N-terminal region that contains an actin-binding module (blue box) and binding sites for the dAbp1 SH3 domain (orange box). The actin-binding module recruits PakB to actin-rich regions at the cell cortex, where it serves as a docking site for dAbp1. Whether the P2 and P3/4 motifs can support the concurrent binding of two dAbp1 molecules is not known. dAbp1 contains an ADF-H domain that weakly binds actin filaments and a P2-P3/4-like sequence (orange) that binds its own SH3 domain, suggesting that dimers or oligomers of dAbp1 may assemble to cross-link and strengthen the local actin network. dAbp1 interacts directly with MyoK, and possibly other class I myosins, suggesting that it may facilitate their recruitment to the complex to be phosphorylated and activated by PakB.