Figure 6. Model for EGL-4 regulation of nociceptive signaling.
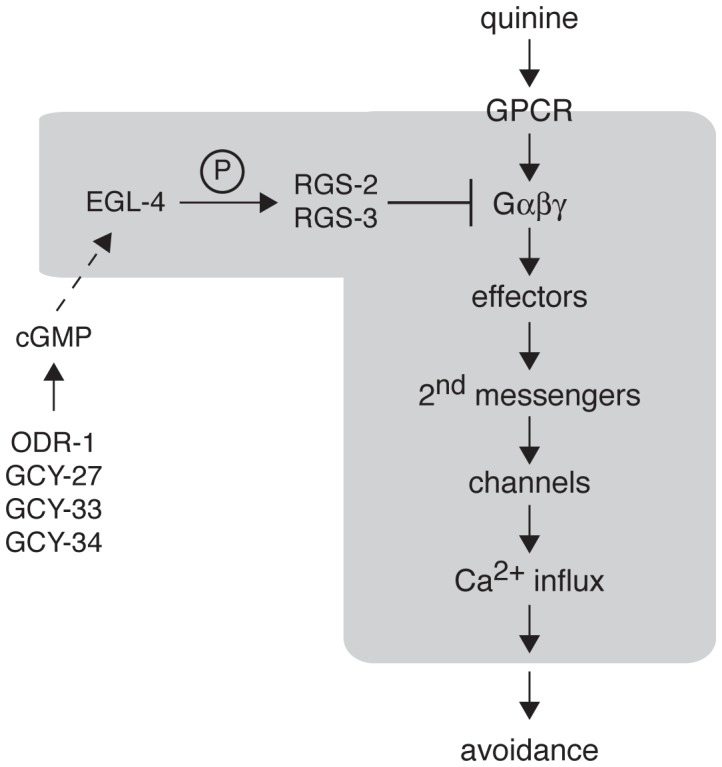
The cGMP-dependent protein kinase EGL-4 regulates C. elegans behavioral sensitivity to the bitter tastants quinine and amodiaquine and the volatile odorant octanol. Wild-type chemosensory signaling is initiated in the ASH sensory neurons when a ligand (such as quinine) binds to a GPCR to activate the associated heterotrimeric G proteins. The activated G proteins (Gα-GTP and Gβγ) interact with downstream effectors to generate second messengers that can activate channels in the plasma membrane, allowing Ca2+ influx. Through connections with downstream interneurons and motor neurons, ASH activation is ultimately translated into behavioral avoidance (backward locomotion). Signaling is terminated in part by regulator of G protein signaling (RGS) proteins, which promote the hydrolysis of GTP to GDP by the Gα subunit. EGL-4 phosphorylation of RGS-2 and RGS-3 stimulates their activity. The guanylyl cyclases ODR-1, GCY-27, GCY-33 and GCY-34 may function in alternate neurons to provide the cGMP that is required for EGL-4 function in ASH. In the absence of EGL-4 function, RGS-2 and RGS-3 do not efficiently downregulate Gα signaling, leading to increased Ca2+ levels in response to receptor activation. This increased signaling in the ASH sensory neurons leads behavioral hypersensitivity to weak stimuli. The molecular events believed to be happening within the ASHs themselves are included within the grayed area.
