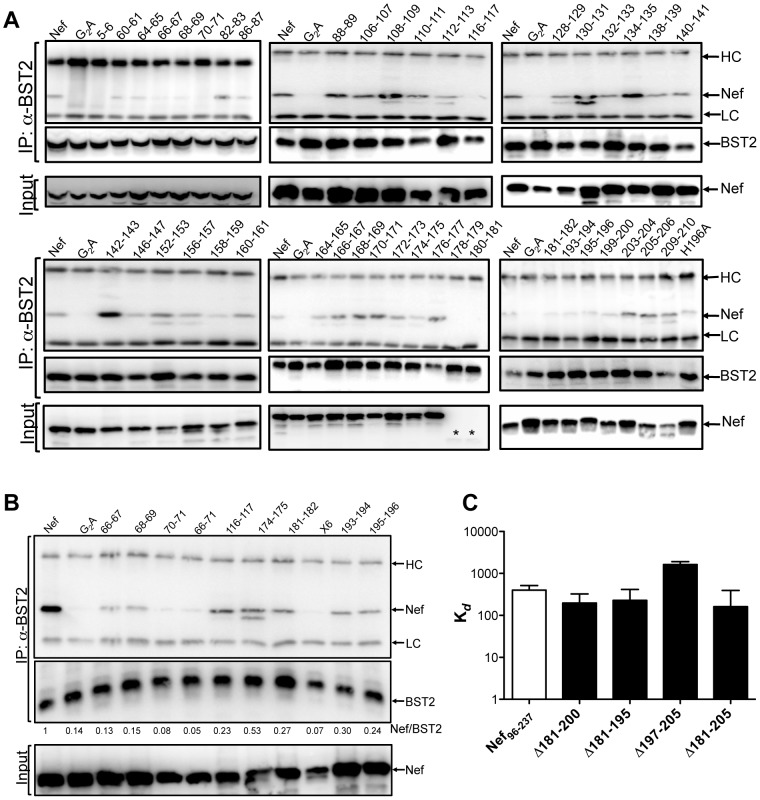
Figure 3. Identification of residues in SIV Nef that contribute to interactions with tetherin.
(A) Co-immunoprecipitation assays with the Nef mutants that impair anti-tetherin activity were performed to identify residues that diminish binding to tetherin. 293T cells were co-transfected with constructs expressing rhesus tetherin, and either wild-type or mutant Nef proteins. Cell lysates were immunoprecipitated using a monoclonal antibody to tetherin, and blots were probed with monoclonal antibodies to SIV Nef and tetherin. (B) Combinations of alanine substitutions in Nef that were shown to impair binding to rhesus tetherin in panel A were tested in additional co-immunoprecipitation assays as described above. (C) Estimated Kd.app values were determined for the binding of SIVmac239 Nef96–237 with the indicated deletions in the flexible loop region to the cytoplasmic domain of rhesus tetherin by SPR. Bands corresponding to the antibody heavy and light chain are indicated (HC and LC). Asterisks indicate the absence of detectable Nef protein.