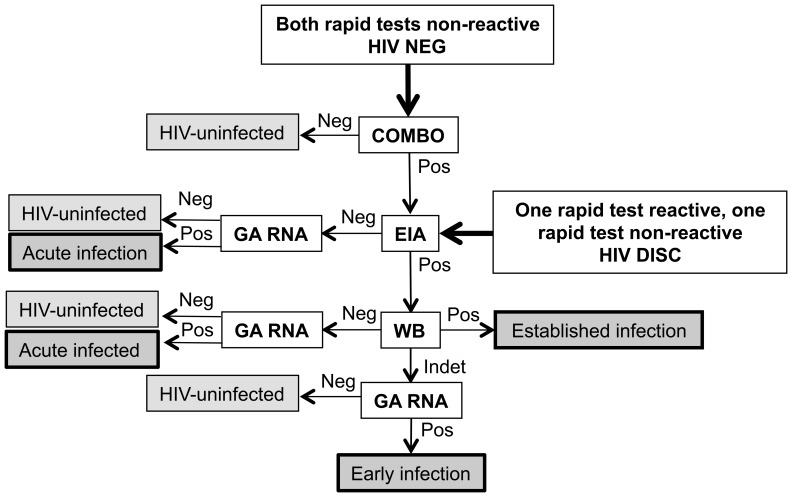
Figure 1. Algorithms used for quality assurance testing of study samples.
The figure illustrates the testing algorithms that were used to determine and/or confirm the HIV status of study samples. This quality assurance testing was performed at the HPTN Network Laboratory (see Methods). The algorithm used for quality assurance testing was determined by results obtained from HIV rapid testing performed at the study sites (for samples initially designated as HIV NEG, HIV DISC, and HIV POS, see Methods). Quality assurance testing was performed for HIV POS samples if results from the avidity assay suggested absent or very low levels of anti-HIV antibodies (weird avidity). In this case, the HIV DISC algorithm was used to determine HIV status. Neg indicates that a negative or non-reactive test result was obtained. Pos indicates that a positive or reactive test result was obtained. Arrows (non-bolded) indicate the next step in sample testing. The following abbreviations were used to describe assays and tests used in the analysis (see Methods): HIV Combo: ARCHITECT® HIV Ag/Ab Combo assay; EIA: Vitros EIA Human Immunodeficiency Virus Type 1 and/or 2 (HIV-1/2) Antibody Detection in Human Serum and Plasma; GA RNA: APTIMA® HIV-1 RNA Qualitative Assay; WB: Genetics System HIV-1 Western Blot.